May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
Thermal conductivity refers to a material’s intrinsic ability to transfer or conduct heat. The thermal conductivity of a material has many implications. It is fundamental in the design, engineering, and application of materials in environments where heat transfer is a critical factor, such as:
Understanding thermal conductivity (TC) is essential for optimizing thermal management systems, enhancing energy efficiency, ensuring safety, guiding material selection, driving innovation, and improving the performance and sustainability of products and processes.
Thermal conductivity (often denoted by k, λ, or κ) refers to a material’s intrinsic ability to transfer or conduct heat. It is also defined as the material plate of unit thickness conducting the amount of heat per unit time per unit area, with its faces differing by one unit of temperature.
It is one of the three methods of heat transfer, the other two being convection and radiation. Heat transfer processes can be quantified using appropriate rate equations. Fourier’s law of heat conduction forms the basis for the rate equation in this mode of heat transfer.
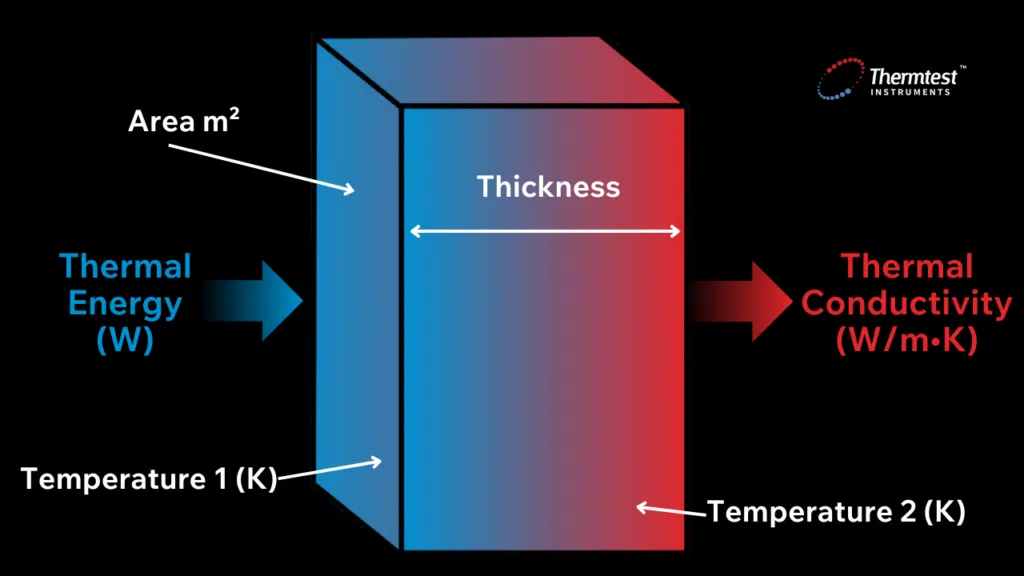
Heat conductivity occurs through molecular agitation and contact and does not result in the bulk movement of the solid itself. Heat moves along a temperature gradient from an area of high temperature and high molecular energy to an area with a lower temperature and lower molecular energy. Heat transfer will persist until both objects reach the same temperature, known as thermal equilibrium. The rate at which heat transfers depends on the temperature gradient’s magnitude and the material’s specific thermal characteristics.
Thermal conductivity is quantified using the International Systems of Unit (SI unit) of W/m•K (Watts per meter per degree Kelvin) and is the reciprocal of thermal resistivity, which measures the ability of an object to resist heat transfer. It can be calculated using the following equation:
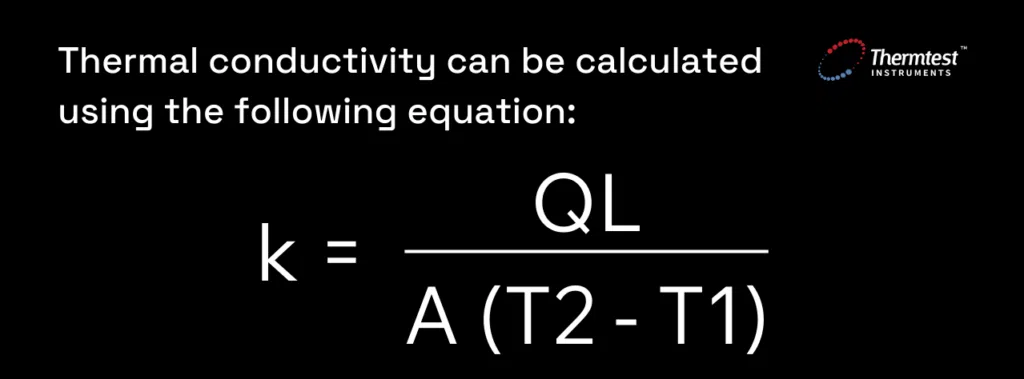
Figure 2. The formula used to calculate thermal conductivity of a sample.
There are two methods for testing the TC of materials: steady state and transient. These methods differ in how they work. Steady-state methods require the sample and reference pieces to be at thermal equilibrium before the measurements begin. On the other hand, transient methods do not need this and can provide results more quickly.
The steady-state technique establishes a constant temperature difference across the sample and measures the resulting heat flow. This method requires achieving thermal equilibrium, where time does not impact or change the temperature of the testing sample.
This method works by applying a known heat flux to your sample and measuring its temperature difference. Once the sample reaches a steady-state temperature (temperature across the sample is constant with no changes recorded over time), then the calculation of thermal conductivity can begin. The steady-state method considers the medium isotropic and assumes one-dimensional heat flow.
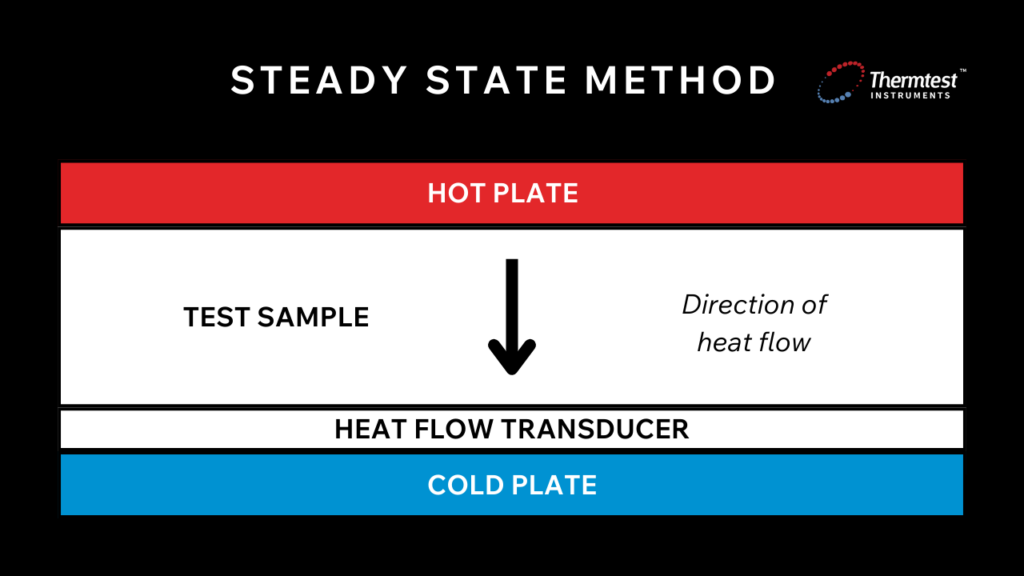
Many methods are considered steady-state techniques, and each has specific configurations and applications. For example, placing a sample between a heat source and a heat sink with a known power output creates a temperature drop across a given sample length, referred to as the absolute technique.
Variables can impact the accuracy of steady-state measurement, including radiative and convective heat loss during setup and errors in the thickness of the sample tested. Steady-state methods are valuable for measuring TC in materials where achieving a steady-state condition is feasible despite potential sources of error. However, utilizing the steady-state method typically requires a very well-engineered setup to perform accurate tests.
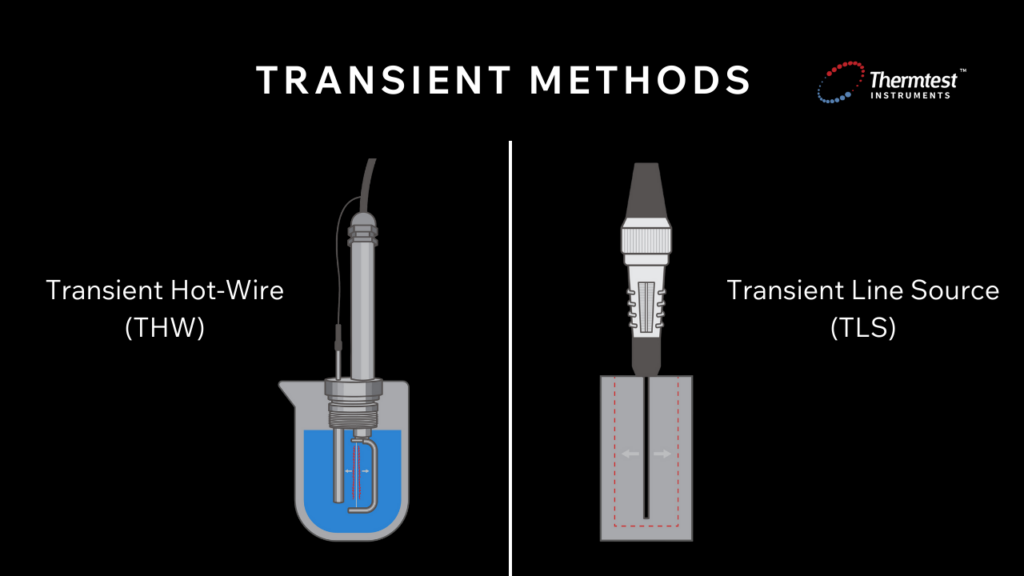
Transient methods determine a sample’s thermal properties by observing how it responds to temperature changes over time.
Unlike steady-state techniques, which require establishing a constant temperature gradient across a sample, transient methods involve applying a heat pulse (or a periodic heat source) to the sample and then measuring the temperature response as a function of time.
One example of a transient method is the Transient Hot-Wire (THW) method, which measures the thermal conductivity of liquids. The THW method involves a thin wire inserted into the sample. This wire is both a heat source and a temperature sensor and when an electrical current passes through the wire, the temperature increases, recording this temperature rise over time.
Another example of a transient method is the Transient Line Source (TLS) method, which determines the thermal properties of solids like rock, concrete, or polymers. Similar to THW, this method involves inserting a probe into a sample. For the TLS method, a needle probe acts as a heat source and a resistance thermometer. While the probe is inserted into the sample, the material is heated for a set time and temperature measurements are taken at constant intervals.
While transient methods allow these measurements to be taken relatively quickly, mathematically analyzing the data from the measurements is more complicated. Transient methods, including the THW and TLS, are favoured for their speed, flexibility, and ability to measure a wide variety of materials, including those that are difficult to handle with steady-state methods.
A sample’s TC can drastically change as its temperature increases or decreases. Since molecular movement is the basis of thermal conductance, a material’s temperature significantly influences thermal conductivity.
Molecules move quickly at higher temperatures, indicating that heat will transfer through the material faster. Understanding the effect of temperature on thermal conduction is critical to ensuring that products behave as expected when subjected to thermal stress, which is especially important when working with products that generate heat, such as electronics, and developing fire and heat protection materials to minimize safety risks.
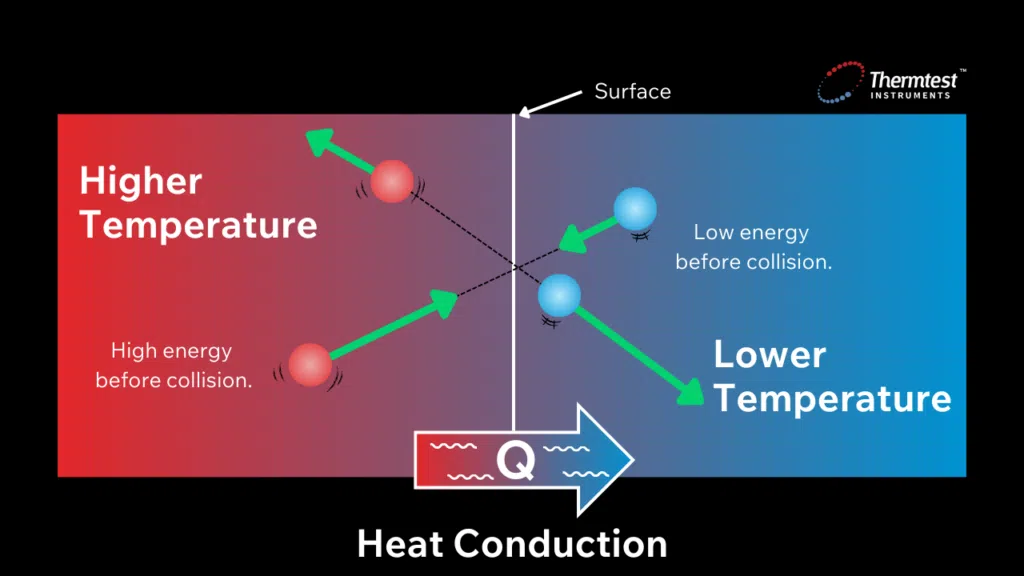
It is essential to understand that the nature of the temperature effect varies across different types of materials. For example;
Metals: Thermal conductivity is primarily due to free electrons in metals. Many pure metals exhibit a peak in TC at very low temperatures. As temperature increases, the thermal conductivity of metals generally decreases because, at higher temperatures, the increased phonon scattering (vibrations within the lattice structure) reduces the mean free path of electrons, thereby decreasing thermal conductivity.
Nonmetals: For non-metallic solids, thermal conductivity is mainly due to phonons. Unlike metals, the TC of nonmetals does not decrease significantly at higher temperatures, except for high-quality crystals at low temperatures where carrier scattering from defects can reduce TC—both thermal conductivity and heat capacity decrease at low temperatures well below the Debye temperature. However, at high temperatures, the TC of nonmetals tends to remain approximately constant.
Gases: Gases’ thermal conductivity increases with temperature because the molecular movement, which is the basis of thermal conductance in gases, becomes more vigorous at higher temperatures. As the internal particle velocity increases, so does the TC, allowing heat to transfer with less resistance. This behaviour is described by the Wiedemann-Franz law, which correlates thermal and electrical conductivity to temperature. However, it’s important to note that the effect of temperature on TC in gases is non-linear and can only be predicted with specific data.
Phase Changes: When a material undergoes a phase change from solid to liquid, the thermal conductivity may change abruptly. For example, when ice melts to form liquid water at 0 °C, the thermal conductivity changes from 2.18 W/(m⋅K) to 0.56 W/(m⋅K). Similarly, the TC of a fluid diverges in the vicinity of the vapor-liquid critical point.
Anisotropic Materials: Due to their anisotropic nature, some substances can exhibit thermal conductivities along different crystal axes. For instance, sapphire has variable thermal conductivity based on orientation and temperature, with different values along the c-axis and a-axis.
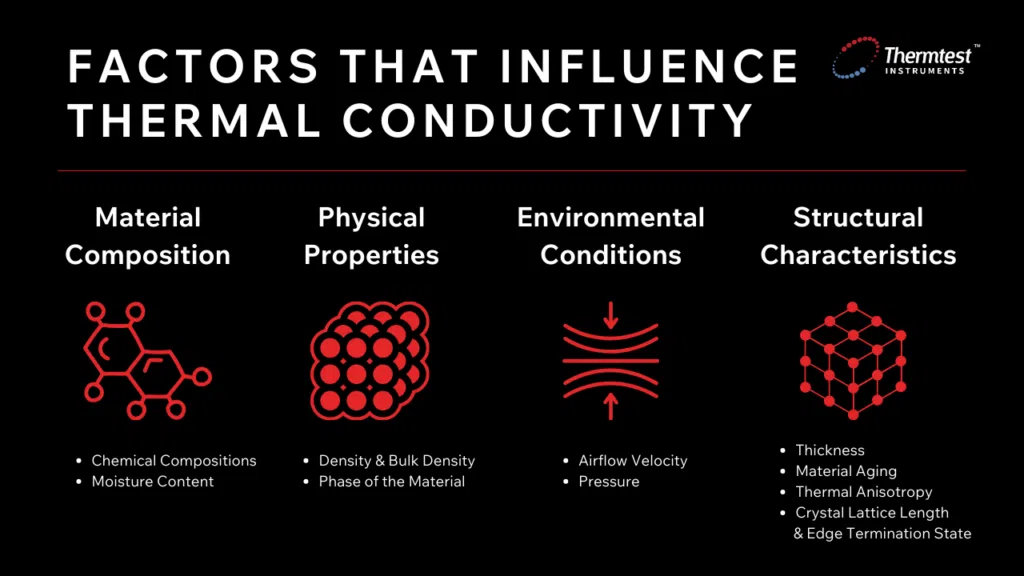
Several factors influence a material’s ability to transfer and conduct heat. These factors can be generally categorized into material composition, physical properties, environmental conditions, and structural characteristics.
Thermal conductivity is a crucial component of the relationship between materials. Effective thermal conductivity testing and measurement are critical to achieving the best possible performance of materials in applications across all industries.
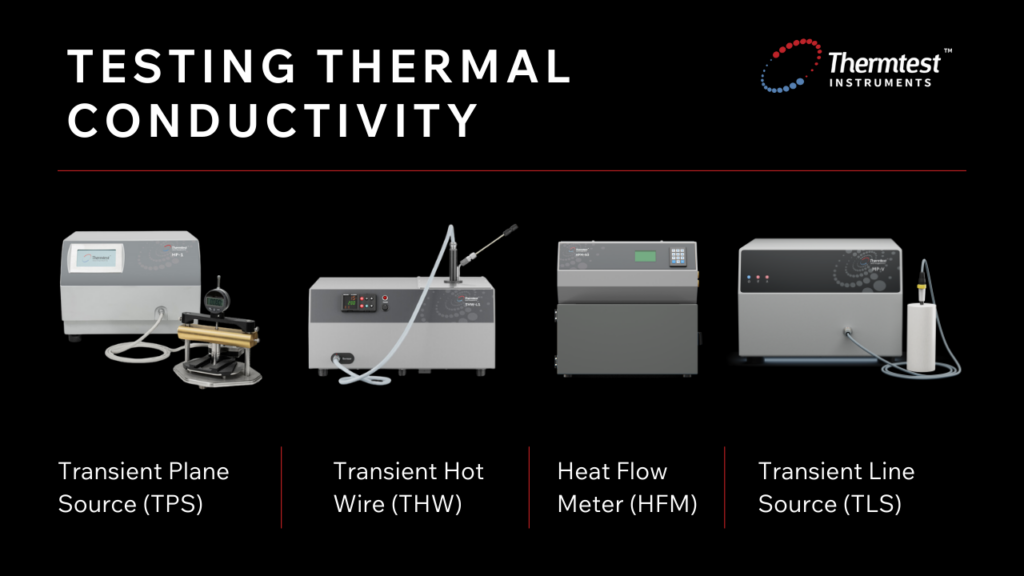
Testing methods are dependent on the material being tested and include:
The transient plane source (TPS) method incorporates a double spiral-shaped sensor that is placed in contact with the surface of the sample material. A heat pulse is sent through the sensor, and the resulting temperature rise is recorded. Based on the rate at which the sensors’ temperature returns to its original state, the thermal properties of the sample are then calculated.
The transient plane source works best when testing solids, pastes, and powders.
A thin heating wire is fully immersed in a small volume of the sample being measured using the transient hot wire method. A current is sent through the wire, which heats the wire and the sample. The electrical resistance in the wire is measured over time, and from there, the thermal properties of the test sample can be determined.
The Transient Hot Wire method tests the thermal properties of liquids and phase-change materials.
Heat flow meter is a steady-state method of determining the thermal properties of insulation and construction materials. This method places the sample between two plates of known temperature difference. The rate of heat flow through the sample is measured, and the TC can be calculated based on the dimensions of the specific sample.
The Heat Flow Meter measures the thermal conductivity and thermal resistance of insulation and construction materials and is best for insulation products, construction materials, packaging, and assemblies.
The TLS method utilizes a long, thin needle or probe that acts as a heat source and a resistance thermometer. The needle is inserted fully into the sample, and the material is heated for a set time while temperature measurements are taken at constant intervals.
During the cooling period, temperature readings are retaken at the same intervals. The TC of the sample is then determined by using the obtained data and the following equation:
k= q/4πa
Where k is the thermal conductivity in W/mK, q is the heating power of the needle, and a is the slope of the line for temperature rise over the log of time.
The desirability of higher or lower thermal conductivity is context-dependent. High TC is beneficial for efficient heat transfer and dissipation. Conversely, low TC is advantageous for insulation and preventing heat loss.
Materials with high thermal conductivity are ideal for applications where heat needs to be moved quickly and efficiently, such as in heat sinks and cooling systems in electronics.
Materials with low TC serve well as insulators for homes and buildings. These materials slow down the transfer of heat, which helps maintain temperature differences between environments.
Thermal conductivity is a common phenomenon that affects our daily activities in ways we may not realize. For instance, let’s say you’re in the kitchen, preparing your morning coffee. You use a metal spoon to add some sugar to your coffee, and then, the doorbell rings. While you’re away, the spoon remains in the cup filled with hot coffee for a few minutes. When you return, the spoon is hot to the touch. This is a typical example of heat transfer via conduction that most of us experience daily.
Specific heat capacity relates to a material’s ability to store heat energy, while thermal conductivity pertains to its ability to conduct heat. Specific heat capacity focuses on the amount of energy required to change the substance’s temperature, while thermal conductivity deals with the rate of heat transfer through the material.
There are multiple factors that contribute to a material’s thermal conductivity. This includes ambient temperature, material composition such as chemical composition and moisture content, physical properties such as density and phase, environmental conditions including airflow velocity and pressure density, as well as structural characteristics such as thickness and material anisotropy.
Diamond currently holds the title for the material with the highest thermal conductivity, with values ranging from 2000 to 2500 W/m·K. This exceptional TC is due to its highly ordered crystal structure and the strong covalent bonds between the carbon atoms in the lattice. Diamond’s ability to conduct heat so efficiently makes it an excellent material for heat sinks and thermal management applications in electronics.
Materials with low thermal conductivity are often used in applications where insulation from heat is required, such as within building materials like insulation, thermal protection systems, and electronic devices to prevent overheating.
The low TC of aerogel is the lowest known to solids and the lowest recorded thermal conductivity of any physical material, with values as low as 0.023 W/m·K. Aerogels are lightweight, low-density materials derived from silica gels in which liquid is absent and ultimately replaced by gas.
This low TC and, therefore, high insulative capability can be attributed to the presence of small, nano-sized pores filled with air, comprising 97% of the material, and small, scattered clusters of silica solids, encompassing the remaining 3%. Aerogels themselves are extremely fragile. However, the blanket-gel combination is a flexible, high-tech insulation that may be used for many applications, such as aerospace, appliances, apparel, and pipelining.
Polyurethane Foam is another material with low thermal conductivity, typically around 0.025 W/m·K, making it an effective insulator. The construction industry utilizes its versatility to fulfill multiple needs, such as insulation, adhesives, coatings and, of course, foam.
Heat transfer rate refers to the amount of heat, Q being transferred per unit time, t. The SI unit of heat is the joule, while the SI unit of time is the second. Thus, heat transfer rate is measured as joule per second (J/s).
Nave, R. HyperPhysics. “Thermal Conductivity”. Georgia State University.
Available at: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thercond.html#c1
NDT Course Material. “Thermal Conductivity”. NDT Resource Center.
Available at: https://www.ndeed.org/EducationResources/CommunityCollege/Materials/Physical_Chemical/ThermalConductivity.htm
Williams, M. “What is heat conduction?”. Phys.Org. December 9, 2014.
Available at: http://phys.org/news/2014-12-what-is-heat-conduction.html
What do you mean by thermal conductivity? Retrieved from Definition of Thermal Conductivity
Thermtest Materials Thermal Properties Database. List of Thermal Conductivities
Redner, S. (2006). Heat conduction. Boston University Physics. https://physics.bu.edu/~redner/211-sp06/class-thermodynamics/heat_conduction.html
Britannica. (2024, April 1). Thermal conduction. https://www.britannica.com/science/thermal-conduction