
November 18, 2024
Discover all there is to know about the principle of heat transfer and how this concept impacts industry and daily life.
Heat transfer can be defined as a branch of thermal engineering that deals with the production, use, change, and transportation of thermal energy from one physical system to another. This process is carried out by means of conduction, convection, and radiation.3
In solids, liquids, and gases, heat is transferred in different forms. This process plays a critical role in various applications, from HVAC systems to electronics cooling, and even in everyday cooking. Understanding this enables improvements in system performance, the extension of energy applications, and research breakthroughs in an array of different fields. In this article, the basic definition will be presented together with other forms of heat transfer and the mathematical principles of these phenomena.
Heat transfer can be described as the process where heat is passed from one substance to another. This process occurs because of a temperature difference between two objects, in which heat transfers from the hotter object to the colder one according to the 2nd law of heat transmission.1 This happens for all three states of matter – the solid, liquid, and gaseous phases – and all exhibit a different efficiency of heat transfer.
In solids, heat mainly transfers through conduction, where molecules of the substance vibrate and pass their energy to adjacent molecules. Liquids and gases transfer thermal energy through conduction and convection, where the warmer parts of the liquid or gas move up and the cooler parts move down, forming a circular current. This can also occur through radiation, where heat is transferred through the emission of electromagnetic waves. Radiation does not necessarily require a medium which means it can happen in a vacuum and through any transparent substance, irrespective of its state.
Understanding heat transfer is crucial to design systems that manage thermal energy efficiently. For instance, in engineering, knowledge of this concept is applied to improve heat exchangers, the insulation of buildings, and the cooling systems of electronics. To achieve these objectives, it is important to accurately measure thermophysical properties with intelligent instruments. Explore Thermtest’s product line to find the right instrument for your specific application requirements.
Heat transfer is of enormous significance in many branches of engineering, focusing on issues of energy application and interaction with the environment. The new methods help minimize energy use in construction and enhance industrial effectiveness and efficiency.
For the environmental field, the right control and use of heat transfer prevents excessive waste heat, which reduces emissions of greenhouse gases. For example, using improved insulation reduces energy consumption by minimizing heat or cooling loss, which lowers the building’s overall carbon footprint. On another level, improvements in technologies for heat transfer result in more efficient engines and industrial processes that further contribute to the saving of energy.

Figure 1: Making heat transfer more efficient helps to reduce greenhouse gas emissions.
Heat transfer occurs in three primary ways: conduction, convection, and radiation. Each method has distinct characteristics and is governed by different physical principles.
Conduction happens when materials or objects are in direct contact with each other. The particles in the warmer object have more energy and vibrate faster than those in the cooler object. The faster vibrating molecules collide with the slower molecules. This makes the cooler molecules vibrate more quickly, which causes the object to warm up. Consequently, the energy transferred from the warmer object causes it to cool. This process occurs until an equilibrium is achieved between both objects.
Convection is the process of heat transfer through the movement of fluid masses, where heat is carried throughout the system by the motion of the fluid. Since heat naturally rises in a fluid with a temperature gradient (where some areas are warmer and others cooler), the warmer, less dense regions will tend to move upward. This upward movement is driven by buoyancy forces, leading to convection, which results in the transfer of heat.
Radiation is the third type of heat transfer. Unlike convection and conduction, radiation does not require a medium to travel; it can move through matter or a vacuum. Thermal radiation is the transfer of energy via electromagnetic waves. A common example of radiation is the sun heating the earth, where the waves travel through space and are absorbed by the earth’s surface. Now, let’s dive deeper into each of these methods.
Conduction is the process where heat transfers from one molecule to another. This phenomenon occurs in gases, liquids, and solids.
For example, heat travels by conduction from a hot cooking tool to your hand, from a soldering iron to the solder, and from the roof of your house to the snow on top of it. Conduction is the only way heat can move through opaque solids.
However, in transparent materials like glass, some heat can also be transferred by radiation. In fluids that are moving, conduction works alongside convection, and if the fluid is transparent, radiation can also play a role.4
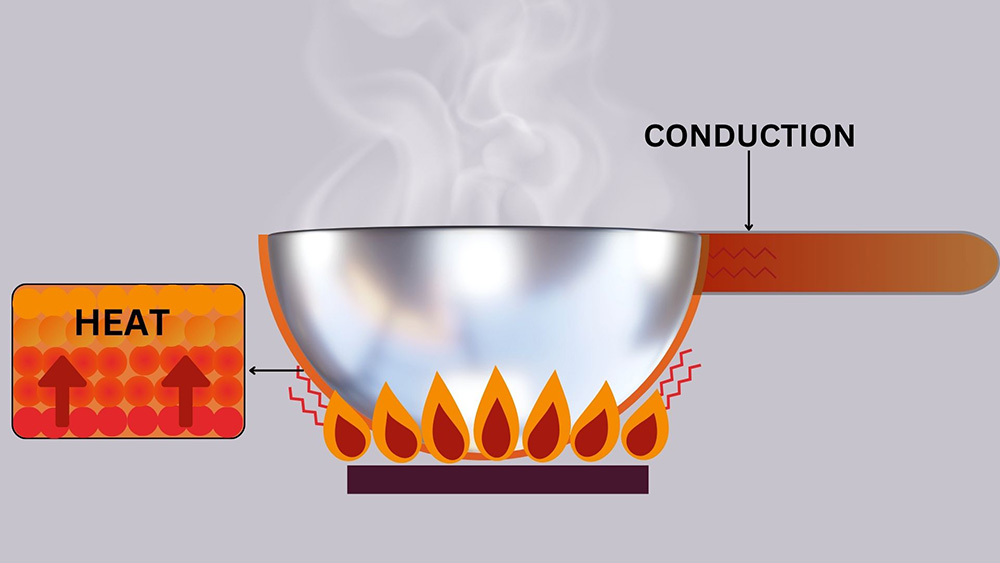
Figure 2: Process of Conduction can be observed with the transfer of heat from the pan to the handle.
In everyday life, conduction can be observed when a metal pan heats up after being placed in a hot liquid. The heat moves from the hot liquid to the pan and then along the pan’s length. Conduction is particularly significant in solids, where molecules are closely packed and can efficiently pass energy to each other.
Explore the examples of heat transfer through conduction in everyday life.
Convection is the way heat is transferred through the movement of fluid molecules. During convection, heat moves with the fluid. When a fluid touches a surface that’s either hotter or colder than the fluid, heat is transferred by conduction from the surface to the fluid (or vice versa). Then, the movement of the fluid spreads the heat within it.
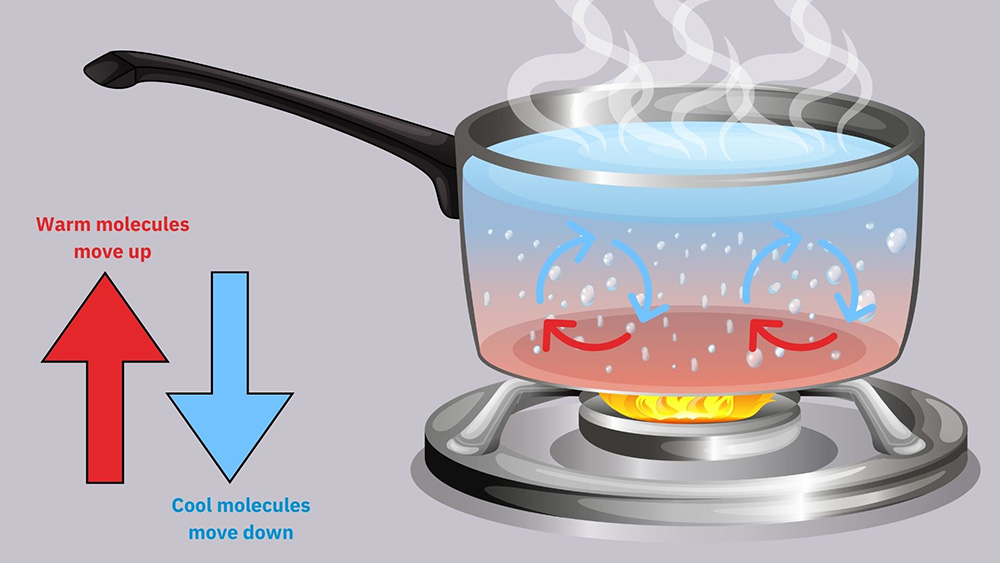
Figure 3: Process of Convection explained by how water is boiled.
For heat to transfer between a surface and a fluid by convection, conduction must happen first at the point where the fluid meets the surface. The fluid’s movement can happen naturally due to differences in density caused by temperature changes (natural convection), or it can be forced by something like a fan (forced convection).
Convection can occur in fluids that are either all in one phase, like just gas or liquid, or in fluids that change phase, like when a liquid turns into a gas. Phase changes can increase heat transfer because extra energy is needed to change the phase, like when water evaporates or freezes. For example, when sweat evaporates from your skin, it helps cool your body down to a temperature lower than the surrounding air.
Convection plays a crucial role in many engineering systems and natural processes. For instance, most of the heat from a steam or hot-water radiator warms a room through convection. In vehicles, the heating and cooling of the passenger compartment is mainly due to convection. When natural gas burns in a water heater, convection transfers the heat to the water tank. In lakes and ponds, water freezes on the surface first because of natural convection. Ice cools a drink partly through convection, and soup in a pot is heated by forced convection when stirred or by natural convection when left unstirred.4
Discover the most common examples of convection heat transfer.
All materials, regardless of temperature, emit radiation in all directions due to the random movements of molecules. These movements involve charged particles like protons and electrons, which emit electromagnetic radiation, carrying energy away from the surface.
Unlike conduction and convection, radiation can transfer heat through a vacuum, making it possible for the energy from the sun to reach the Earth. Radiative heat transfer occurs between surfaces at different temperatures and can be absorbed, reflected, or transmitted. The effectiveness of a material in emitting, absorbing, or reflecting radiation depends on its surface properties, which are described by terms like emissivity and reflectivity.
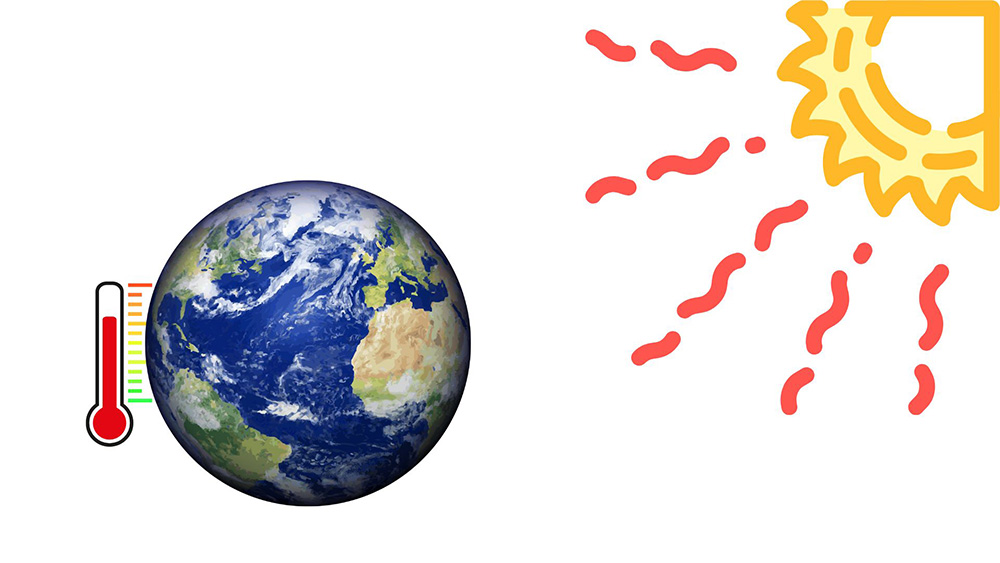
Figure 4: The sun radiates thermal energy to the earth, keeping it warm.
Thermal radiation is absorbed well by materials such as water, water vapor, glass, wood, brick, stone, concrete, asphalt, and copper. On the other hand, materials like aluminum foil reflect thermal radiation effectively.1
Liquids and gases interact with radiation differently depending on the wavelength. Some wavelengths pass through them easily, while others are absorbed. For example, greenhouse gases like carbon dioxide and methane are highly effective at absorbing infrared radiation, contributing to global warming.4
Explore real-world examples in our article on examples of radiation heat transfer.
The mathematical modeling of heat transfer is pivotal for predicting the thermal behavior of systems. Fourier’s Law of Conduction and Newton’s Law of Cooling are fundamental equations applied to all three mechanisms. These equations help in calculating the rate of heat transfer, which is essential for designing thermal systems.
This law states that the rate of heat transfer through a material is proportional to the negative gradient of the temperature and the area through which the heat is flowing. The basic one dimensional equation is:
\[ q=λ A \frac{dT}{dx} \]
where:
This law describes the rate at which an exposed body changes temperature through convection, which is proportional to the difference in temperature between the body and the surrounding environment. The equation is:
\[ q=h A (T_{s}{-}T_{∞}) \]
where:
Understanding these equations allows engineers and scientists to predict how heat will move through different materials and environments, enabling the design of more efficient and effective systems. Check out this heat transfer calculator for convenience.
Understanding the basics of heat transfer is crucial for both everyday life and industrial applications. Heat transfer occurs in three primary ways: conduction, convection, and radiation. Each method operates under different physical principles and plays a significant role in various processes.
Conduction occurs through direct contact, where energy is transferred from one molecule to another. Convection involves the movement of fluids, where heat is transferred as warmer fluid rises and cooler fluid sinks. Radiation, unlike conduction and convection, transfers energy through electromagnetic waves.
The significance extends beyond basic physics; it is fundamental in engineering fields, particularly in improving energy efficiency and reducing environmental impact. Effective heat transfer management can lead to optimized industrial processes, better building insulation, and more efficient engines, all of which contribute to sustainability by reducing energy consumption and minimizing waste.
Physical laws, such as Fourier’s Law of Conduction and Newton’s Law of Cooling, provide the tools necessary for engineers and scientists to predict and control heat transfer in various systems. These equations are essential for designing systems that manage thermal energy effectively.
In conclusion, mastering the principles of heat transfer is essential for developing efficient, sustainable technologies that enhance both industrial processes and everyday life.5
Heat transfer is the process by which thermal energy moves from a region of higher temperature to a region of lower temperature. This can occur through conduction, convection, or radiation.
The three types are conduction, convection, and radiation. Conduction involves direct transfer through materials; convection involves the movement of fluids; and radiation involves the transfer of heat through electromagnetic waves.
The heat transfer coefficient can be determined experimentally or through empirical correlations. It is a measure of how well heat is transferred between a solid surface and a fluid, and it varies depending on the fluid and the surface, and the flow conditions.
Heat transfer can be calculated using various equations, such as Fourier’s Law for conduction and Newton’s Law of Cooling for convection. These equations consider factors like thermal conductivity, surface area, temperature difference, and heat transfer coefficient.
1Heat transfer: Conduction, Convection & Radiation. (n.d.). Retrieved from GreenSpec.co.uk: https://www.greenspec.co.uk/building-design/heat-transfer-conduction-convection-radiation/
2Introduction to Fourier’s Law. (2020). Retrieved from innovationspace.ansys.com: https://innovationspace.ansys.com/courses/wp-content/uploads/sites/5/2020/03/Lesson-1-Introduction-to-Fouriers-Law.pdf
3Introduction to Heat Transfer. (2021). Retrieved from Let’s Talk Science: https://letstalkscience.ca/educational-resources/backgrounders/introduction-heat-transfer
4Luebbers, M. W. (2024, August 09). Heat transfer. Retrieved from Access Science: https://doi.org/10.1036/1097-8542.311100
5Thermal diffusivity. (n.d.). Retrieved from Mordern Physics: https://modern-physics.org/thermal-diffusivity/