May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
The design of this experiment is to understand why a piece of metal will FEEL colder, while a piece of wood or plastic will FEEL warmer when they are both at the same temperature. To visualize the process of heat transfer, a free thermal simulation software will be used.
We have this experience every day, one example is when we step out of a warm area, such as a shower, and have the choice to sit in a metal chair and a cushioned chair, cushioned chair will be chosen. The first thought that will come to mind is that the metal chair is much colder than cushioned making it an uncomfortable choice, however; the fallacy is that both chairs (if kept in the same room) are the same temperature. This phenomenon is due to the difference in thermal conductivity of both materials
Thermal Conductivity is a measure of a material’s ability transfer heat and is directly proportional the rate of heat transfer. Metals are among the better thermal conductors, whereas; Non-Metals are among the better thermal insulators.
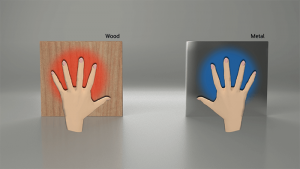
As seen in the video, heat will travel faster through a metal as compared to a non-metal. In the example, for a person touching a piece of metal, heat is being removed from their hand at a much faster rate as compared to piece of wood or plastic. Effectively, the metal is removing heat from your hand faster than your body heat can replace it, therefore; the temperature of your hand is decreasing. This decrease in hand temperature causes the thermoreceptors in your hand to FEEL the metal as being cold.
There is actually a specific property that governs this, thermal effusivity. It is a combination of thermal conductivity and of how much heat something can absorb (heat capacity). It determines the temperature at the surfaces of two objects touching. Touching an object with high effusivity, like metal, will make the temperature of your skin drop close to the object’s temperature, while touching an object with low effusivity, like wood, will have little effect on your skin.
In the previous un-altered example, the properties of the materials used were not exactly mirroring the properties of wood and metal. The thermal conductivity of the wood is set at 0.1 Watts per meter Kelvin (W/m-K) and the metal at 1 Watts per meter Kelvin (W/m-K). The properties of these materials can easily be modified via a Right click -> Properties -> Thermal OR the Thermtest Thermal properties database can be searched in the red box below.
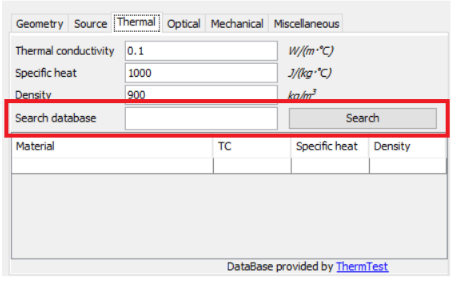
TC – Thermal Conductivity (W/m⋅K)
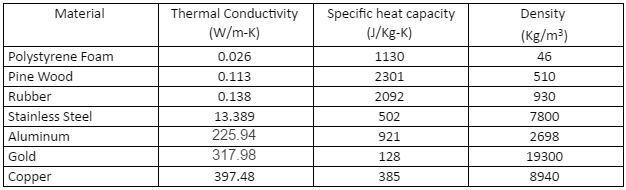
Above is a list of properties of some metals and non-metals which can be used to visualize a more realistic transfer of heat. More properties can be found here, simply type in the material you wish to mimic or pick from the 50 most commons materials.