May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
When one thinks of thermal conductivity, the term is almost always thought to be associated with solids such as Copper, Silver and so on, however; there exists Thermal Conductivities associated with fluids (liquids and gases). Albeit the values of thermal conductivity of liquids and gasses are very small and hard to precisely (and practically) calculate due to convection (for an experiment explaining convection this see our balloon experiment), a qualitative comparison can easily be done. Using common cooking supplies, a comparison can be made by simply heating a container(s) containing each liquid and monitoring the change in temperature. For specific vales for the thermal conductivity of liquids check out our Materials Database
The goal of the experiment is to gain a better understanding of thermal conductivity and how this concept applies to fluids. This concept is quite important as it plays an important role in heat management, electronics and various other industries.
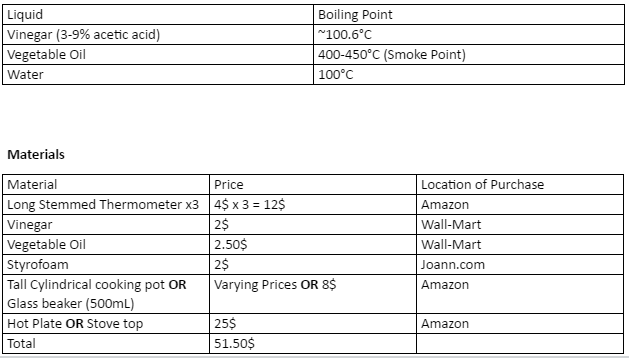
It is important to take all necessary safety precautions while performing this experiment and it is important to know the boiling point of each liquid before advancing. Below is a list of the boiling points of the various liquid used in this experiment. If at any moment the liquid within the container begins to boil, remove the container from the heat and remove the Styrofoam lid.
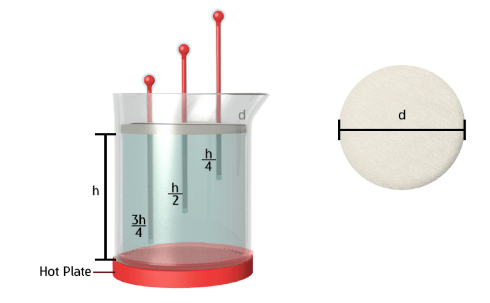
Upon completion of this experiment and the data recorded, an idea of which liquid is the better thermal conductor can be formed. By plotting the graph of temperature against distance, the liquid with the greatest difference between ¾ distance and ¼ distance will be the worst thermal conductor. Hypothetically, since vinegar is mainly 3-9% acetic acid and the rest water, the thermal conductivity of both vinegar and water would be similar. Vegetable oil consists of mainly non-metals (hydrocarbons). Since non-metals are poor thermal conductors it may not be a stretch to hypothesize that vegetable oil will be the worst thermal conductor.
Below is a list of thermal conductivities of various compounds found in common household liquids. These values can be used to further this experiment or compare results obtain while performing this experiment. Included with these values is the boiling point of each liquid.

As well as having a high specific heat capacity, water also holds fairly high thermal conductivity when compared to the set of thermal conductivity of liquids that were tested. This phenomenon is most likely due to hydrogen bonding, keeping water molecules close to one another allowing for more efficient energy transfer. Water does, however; not have the greatest thermal conductivity of liquids. Some liquids and gels have been designed to have thermal conductivities over 6.0 W/m-K (10x that of water). These liquids and gels are known as thermal interface materials and play a very important role in electronics.
For further information, visit: