May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
The following is a simple and inexpensive experiment that you can do by yourself or with a class to investigate how heat is transferred through differing materials, in this case water and air. This is a great way segue into topics such as thermal conductivity, and thermal diffucivity. If you would like to know the thermal conductivity of water, the thermal conductivity of air, or the thermal properties of other materials check out our materials database.
This experiment highlights the difference in the thermal conductivity of air and the thermal conductivity of water, or the absorption and transfer of heat in water as compared to air.
Heat transfer in liquid and gasses is through convection and water absorbs much more heat than air (Holman,1981).
In physical science, heat transfer often refers to the the process by which matter exchanges thermal energy. As such, there are three key ways through which heat energy is transferred between matter.
Materials that you will require for this experiment will include the following:

With all the requirements set, the procedure for the experiment will progress as detailed below:
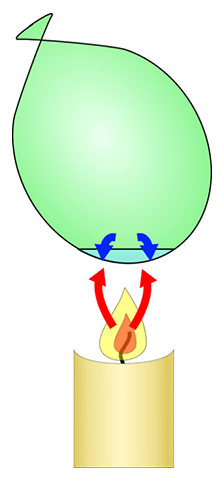
When the balloon filled with air touches the flame, it bursts. However, the balloon that contains water does not burst upon touching the flame.
The balloon filled with air bursts since the air expands quickly and does not absorb the heat from the rubber which causes the rubber ball to stretch and eventually brake to let the expanded air out. The balloon having water and air does not burst. It is because water absorbs the heat from the rubber band and through convection currents it carries the heat away from the rubber while cold water replaces the risen water (Holman,1981).
The conclusions drawn from this experiment confirm that water and air are both heated through convection current, nevertheless the thermal conductivity of water is higher than the thermal conductivity of air. Water absorbs far more heat than air making the balloon heat proof. As such, water can be used as a heated tank.