May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
Graphene is an individual layer of a honeycomb lattice of carbon atoms which makes up graphite and is used for a variety of purposes including electronics, semiconductors and batteries. Its presence is known in these industries due to it having a thermal conductivity constant (k), which is a measure of how fast heat is transferred, of near 1000 W/m*K. Other materials such as cardboard and plastic do not find themselves in these industries due to there lower thermal conductivity constant of around 0.1 W/m*K and 0.2 W/m*K
Graphite will transfer significantly more heat over time from your hand to the ice and thus cut through ice significantly faster when compared to cardboard or plastic.
Graphite sheet should cut through the ice almost instantaneously whereas the cardboard and plastic do not cut through the ice as readily. Image
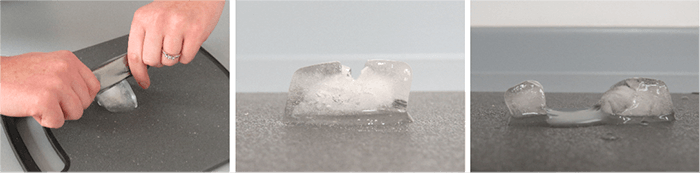
Graphite sheet has a thermal conductivity constant around 10,000 to 5,000 times higher than cardboard and plastic. Therefore, Graphene will transfer significantly more heat over time from a source of heat (your hand) to the ice as compared to cardboard and plastic. Cardboard and plastic do not have such a high thermal conductivity constant and will require more time to transfer the heat from your hand to the ice.
It can be concluded from this experiment that graphite sheets are a significantly higher thermal conductor when compared to both cardboard and plastic.