May 3, 2024
July 7, 2022
June 24, 2022
January 24, 2022
October 28, 2021
July 8, 2019
November 26, 2018
September 22, 2017
June 9, 2017
September 27, 2017
July 24, 2017
September 22, 2017
September 27, 2017
November 26, 2018
January 30, 2019
George Frederick Charles Searle was born December 3rd, 1864 and was a British Physicist and Lecturer. Searle first began working at the University of Cambridge in 1888 at the age of 24 under the supervision of J. J. Thompson. He continued his work for another 55 years. Searle is best known for his contribution to the research on the velocity dependence of electrostatic mass which played an important role in the formulation of Einstein’s special theory of relativity. Along with these achievements, the Searle’s Bar experiment was produced, a simple and affordable way to calculate the thermal conductivity of good thermal conductors.
It is important to note that for this experiment to be successful, the rod being measured must be a good thermal conductor.
Most metals are considered good thermal conductors thus any metal rod would be suitable for this experiment. Common materials with high enough thermal conductivities include: Copper, Stainless Steel and aluminum.
Specific Heat of Water = 4.18 Joules/(gram⋅°K)
Energy absorption of water (Q) = c ⋅ m ⋅ ΔT
Heat Flow Rate (Q) = k ⋅ A ⋅ (T1-T2) / d
Cross sectional Area = πD2 / 4 OR πr2
| Materials Required | Price | Location of Purchase |
|---|---|---|
| Copper Tubing | $7 | Amazon.com |
| Steam Chamber/Maker | Varying Price | Varying Locations |
| Thermometer x4 | $10 x4 = $40 | Amazon.com |
| Beakers (500ml)x2 | $4 | Amazon.com |
| Tubing | $3 | Amazon.com |
| Insulating Material(Cotton or Felt) | $10 | Amazon.com |
| Vernier Caliper | $10 | Amazon.com |
| Metal Rod | Varying Price | Speedymetals.com |
| Digital Scale | $15 | Amazon.com |
| Total | $98 |
*For how to make your own Steam Chamber, See Lee’s Disc Experiment*
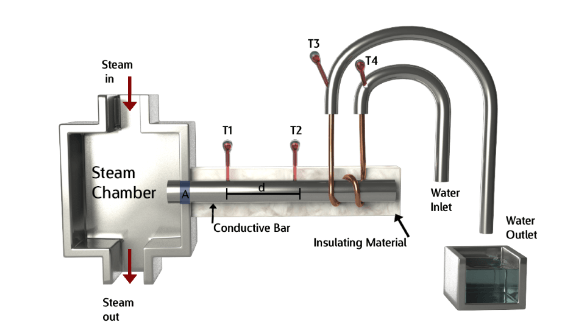
The thermal conductivity apparatus resulting setup should resemble the above figure.
The Rate of Heat transfer between thermometers 1 and 2 over a given distance d will be given by the formula:
This Formula can be rearranged to produce the following:
The amount of energy absorbed by the water is given by:
With this formula in mind, Q of equation (2) can be replaced by the equation (3) producing the following:
A simple insertion of the values experimentally obtained can be performed to calculate the thermal conductivity of the metal in question
Once calculated, the experimental thermal conductivity constant of a given material can be compared to the experimental thermal conductivities obtained by highly specialized labs.
*Thermal Conductivity values taken from the Thermtest thermal properties database, for more Thermal Conductivity values visit: https://thermtest.com/materials-database
Unlike the Lee’s Disc experiment, the Searle’s Bar experiment is intentionally designed to calculate the thermal conductivity of materials with high thermal conductivities. Metals are generally considered the better thermal conductors due to their highly order molecular arrangement however there are some non-metals which can surpass metals in thermal conductivity such as Graphene.
Using this list of instructions, anyone can become a thermal physicist and calculate the thermal conductivity of various metals for an affordable price.
Web Resources for further Information:
https://thermtest.com/thermal-resources/build-your-own/lees-disc/
https://thermtest.com/applications/graphite-sheets-thermal-conductivity/
https://en.wikipedia.org/wiki/Searle%27s_bar_method
To make your own simulation:
https://thermtest.com/thermal-resources/energy2d-heat-transfer-simulations/
| Type of Material | Thermal Conductivity Constant (W/mK) |
|---|---|
| Copper | 397.48 |
| Aluminum | 225.94 |
| Bronze | 188.28 |
| Iron | 71.965 |
Thermtest is furnishing this item "as is". Thermtest does not provide any warranty of the item whatsoever, whether express, implied, or statutory, including, but not limited to, any warranty of merchantability or fitness for a particular purpose or any warranty that the contents of the item will be error-free. In no respect shall Thermtest incur any liability for any damages, including, but limited to, direct, indirect, special, or consequential damages arising out of, resulting from, or any way connected to the use of the item, whether or not based upon warranty, contract, tort, or otherwise; whether or not injury was sustained by persons or property or otherwise; and whether or not loss was sustained from, or arose out of, the results of, the item, or any services that may be provided by Thermtest.