
June 7, 2019
The global plastic industry took off and has continued to grow exponentially since the debut of the first synthetic plastic, Bakelite, in 1907. Synthetic plastic is undeniably one of the most convenient creations known to mankind. It is used daily for a variety of purposes in households across industrialized nations. Products such as disposable dippers, plastic bags and water bottles have all helped lead to the rapid increase in manufacturing plastic products. Industries, construction companies, hospitals and food transportation have a heavy reliance on plastic for their businesses and services to function properly. As a first world society we have become extremely reliant on plastic and its basic chemical properties to help our daily systems operate effectively.

Figure 1: Plastic bottle litter1
The benefits of something as simple as plastic are huge, however, our over-use and incorrect disposal of plastic is leading to a negative effect being inflicted on our planet. This problem isn’t going to disappear anytime soon as plastic patches floating in the ocean and piles building up on city streets continue to grow larger. The convivence and durability of plastic is the root of the problem as it is being mass produced at alarming rates and there are very few proper recycling systems in place on a global scale that are equipped to handle the volume of plastic that is being disposed of everyday.
Globally an average of 260 million tons of plastic are produced annually with 10% of that ending up in the ocean. Plastic is an unique mixture of harmful chemicals, many of which have shown to be carcinogenic leading to multiple types of cancer. The main method for decomposing plastic is by UV light or from exposure to sunlight. However, plastic never really decomposes, it only breaks down into smaller and smaller pieces of plastic, so the chemical composition of each individual piece remains the same. Even though the substance is smaller it still inflicts the same harmful effects if exposed to a larger volume. These so called “degraded” tiny pieces of plastic are flowing through our ecosystems and causing tremendous harm to the terrestrial and aquatic wildlife that they are encountering.
Some of the decomposition lifetimes for certain varieties of plastic surpass 600 years. Hundreds of years are needed for decomposing a single plastic water bottle. How crazy does that seem? So, what is the solution to this over-abundance of plastic circling our globe and oceans? This is where thermal conductivity combined with nature comes into play.
| Type of “Everyday Plastic” | Decomposition Rates in a Coastal Environment |
|---|---|
| Foamed plastics cups | 50 years |
| Plastic beverage holder | 400 years |
| Disposable diapers | 450 years |
| Plastic water bottles | 450 years |
| Fishing line | 600 years |
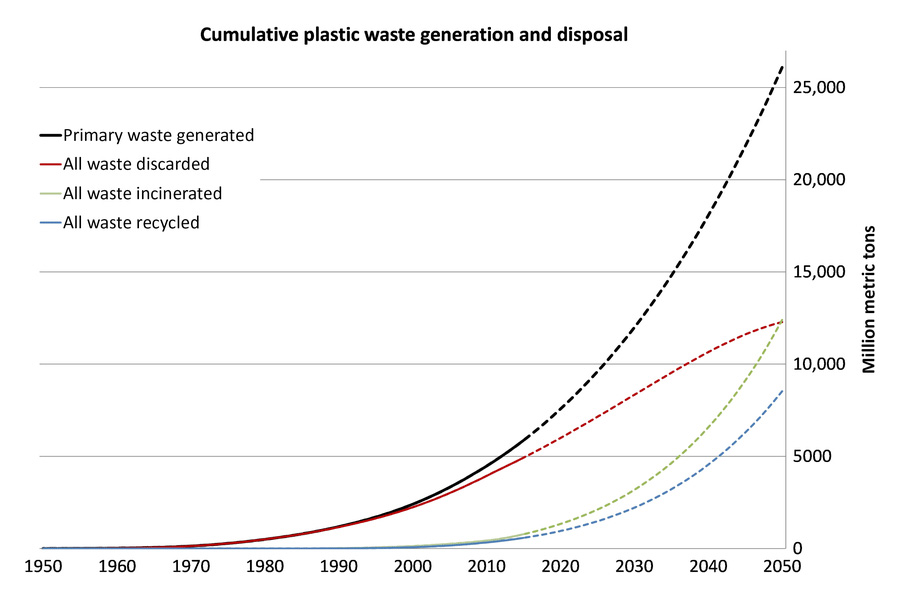
Figure 2: Cumulative plastic waste generation and disposal (in million metric tons).2
Another extremely abundant substance on earth is cellulose. Cellulose is the most abundant natural polymer on earth and can be found in plant cells and is normally located in their cell walls. It is often used in electronics and for aerospace technology because of its strength, toughness and transparency. Researchers Zhang et al are designing a technique that harnesses these useful properties of cellulose to create a cellulose bioplastic constructed from a hot-pressing technique. Cellulose bioplastic (CBP) has extremely high tensile strength, thermal stability, biodegradable and renewable. These are all desired traits for designing an eco-friendly replacement to plastic.
The Cellulose was combined with graphene (a substance with excellent thermal conductivity), to create a novel green cellulose/graphene mixture. None of these substances are toxic to humans or wildlife and can easily be integrated back into natural ecosystems through decomposition. A graphene addition can incorporate many additional benefits that conventional plastics lack.
Due to graphene’s high thermal conductivity it can be used as a modifier in the design of plastics for specific application. Its thermal conductivity properties provide efficient heat transfer from heating and cooling surfaces. Graphene bonds almost pair perfectly to cellulose due to their chemical composition so even when combined they create a homogeneous (uniform) mixture. Due to their near-perfect bonding the cellulose/graphene mixture can retain the transparency trait of cellulose while utilizing the thermodynamically favorable traits of the graphene.
The main challenge researchers are facing involving the combination and mixing of the graphene and cellulose is managing to make the plastic thin enough to compete with products such as cling wrap that are extremely popular in North American markets. Advances and adaptions are being made to achieve the desired thinness to replace products like cling wrap which just happens to be one of the most environmentally harmful common plastic on the market today.
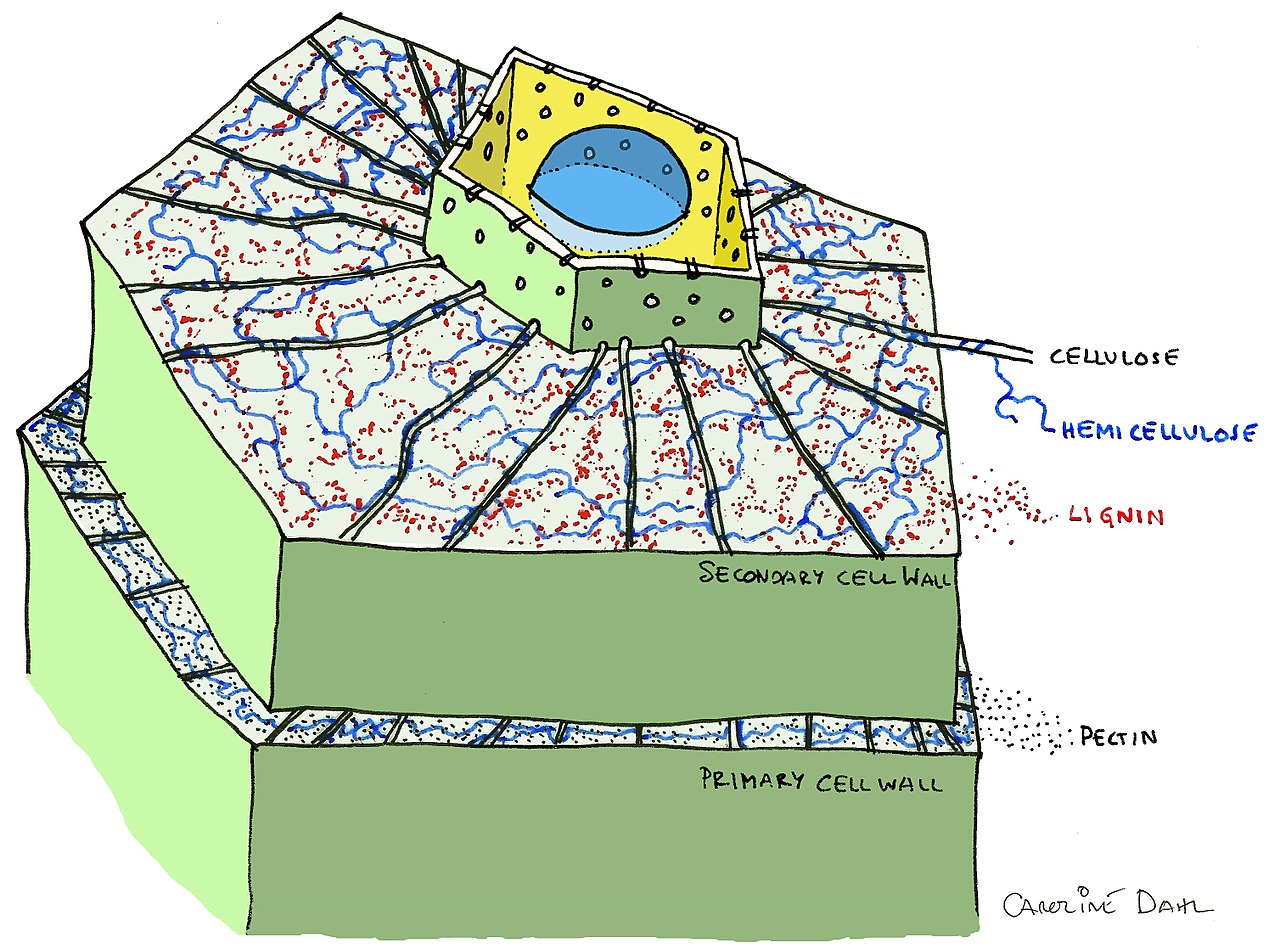
Figure 3: Labelled diagram of a plant cell wall including cellulose3
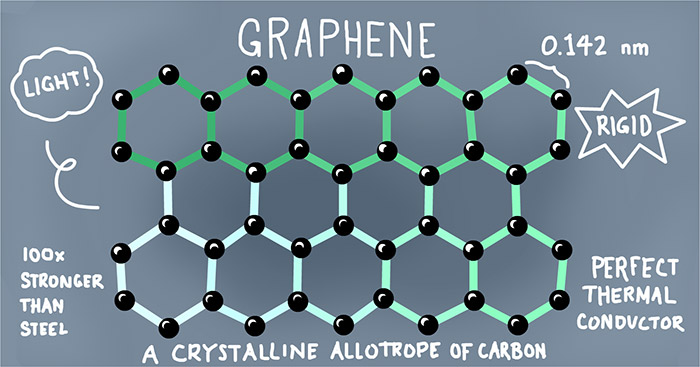
Figure 4: Labelled diagram of the bonds present in graphene molecules4
The raw materials cost for the cellulose substance are higher than conventional plastics but the long-term expenses for production companies will decrease during wide spread practical production. These plastics can be constructed at a temperature averaging between 70-100 degrees Celsius which is substantially lower than 130 degrees Celsius which is the temperature needed to construct polypropylene (the industry’s leading thermoplastic). Due to a lower production temperature that is required for CBPs, factory emissions will also decrease if more industries switch to making CBP instead of your average thermoplastic. In turn, less non-renewable fossil fuels will need to be used to create such a high temperature environment to produce thermoplastics.
The main selling factor of CBP is its biodegradability. It is a substance that originated from nature and can easily be reincorporated back into natural systems. The decomposition lifetime is much shorter and once it is decomposed back into nature, its chemical components can be harnessed by growing plants to make more cellulose which can then be used for more CBP production. This positive feedback loop would only continue to grow stronger as eco-friendly plastic production continued to increase.
CBP products are still being tested and researched to ensure maximum effectiveness and efficiency so they have yet to enter the consumer market. Advances in similar technologies are helping to create a promising future for our planet. Change needs to occur at a rapid rate to reverse the damages humans have inflicted on this planet especially involving over production and misuse of products such as harmful plastics. Supporting green companies and technologies is one of the best things we can do as consumers to create a healthier planet. A green plastic is just the beginning to one of many necessary changes that need to occur in our society to protect something as precious as our environment.
Author: Kallista Wilson, Junior Technical Writer at Thermtest
Applications and societal benefits of plastics. (n.d.). Retrieved from https://royalsocietypublishing.org/doi/full/10.1098/rstb.2008.0304#d3e715
When The Mermaids Cry: The Great Plastic Tide. (n.d.). Retrieved from http://plastic-pollution.org
Song, N., Hou, X., Chen, L., Cui, S., Shi, L., & Ding, P. (2017). A green plastic constructed from cellulose and functionalized graphene with high thermal conductivity. Acs Applied Materials & Interfaces, 9(21), 17914-17922. doi:10.1021/acsami.7b02675
Staff, C. M. (n.d.). Everything You Need To Know About Polypropylene (PP) Plastic. Retrieved from https://www.creativemechanisms.com/blog/all-about-polypropylene-pp-plastic
1 L, I. (n.d.). Sticle de plastic reciclate Poza gratuite – Public Domain Pictures. Retrieved from https://www.publicdomainpictures.net/ro/view-image.php?image=137354&picture=sticle-de-plastic-reciclate
3 Plant Cell Wall Diagram. (n.d.). Retrieved from https://commons.wikimedia.org/wiki/File:Plant_cell_showing_primary_and_secondary_wall_by_CarolineDahl.jpg
4 Graphene. (n.d.). Retrieved from https://www.sketchport.com/drawing/6730621355294720/graphene
Featured Image: Pollution, Plastic, Plastic Waste. (n.d.). Retrieved from https://pixabay.com/photos/pollution-plastic-plastic-waste-4110882/