
May 22, 2020
Metals are typically known to be highly efficient thermal conductors.
This article will explore the mechanisms of heat transfer, what makes metals ideal thermal conductors, and uses of common metals & alloys.

Image 1. A

Image 1. B
Image 1. A and B show visual illustrations of individuals in the kitchen making use of kitchen supplies.
Cooking is a part of everyday life for most people. Hence, cooking appliances are designed with the incentive of ensuring maximum safety and efficiency. This requires an understanding of thermal physics. There is a reason why the heating element of a toaster is typically made of nichrome-wires, mixing spoons tend to be wooden and the material construction of oven mitts would never involve a metal compound.
It is necessary to recall the definition of temperature to understand thermal conduction mathematically.
The operational definition of temperature is the value measured with a thermometer which simply measures the expansion of volume of Mercury.
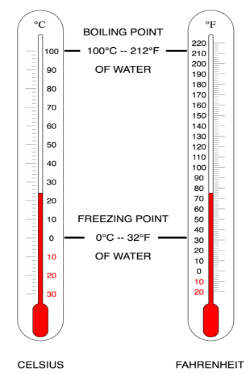
Image 2. Illustration of two thermometers in Celsius and Fahrenheit units
In thermal physics, temperature and thermal conduction are understood through studying the movement of molecules.
Schroeder, the author of ‘Introduction to Thermal Physics’ describes temperature mathematically as:
where:
S=entropy,
U=energy,
N=number of particles,
V=volume of the system (Schroeder, 2007).
Therefore, the temperature of a system is dependent on entropy and energy when the number of particles and the volume of a system is held constant.
Schroeder states in words: “Temperature is a measure of tendency of an object to spontaneously give up energy to its surroundings. When two objects are in thermal contact, the one that tends to spontaneously lose energy is at the higher temperature” (Schroeder, 2007). This is because the two objects in contact will try to reach thermal equilibrium; become the same temperature.
To visualize temperature and thermal conduction at a microscopic level Figure 1 A and B are demonstrated below. Imagine that an unknown object A and B are in physical contact with one another. Object A has a higher temperature than object B. What will happen to the temperature over a duration of time?

Figure 1. A
Figure 1.B
Figure 1.A illustrates two unknown objects in physical contact with each other, and Figure 1.B displays the molecules of the objects.
At t0, TA > TB
At t1, TA > TB
.
.
At tn, TA = TB
At t0, ŝA > ŝB
At t1, ŝA > ŝB
.
.
At tn, ŝA > ŝB
Given that tn: a point in time, TA: temperature of object A, TB: temperature of object B, ŝA: average speed of A particle, ŝB: average speed of B particle.
At t0, the atoms of object A are moving at a faster speed, and atoms of object B are moving at a slower speed (TA > TB). Over time, object A gives up energy and object B gains energy until the they are the same temperature (TA = TB) and reach thermal equilibrium. This is thermal conduction described at a molecular level. The closest atoms of object A bumps into atoms of object B. The atoms of object B that had the initial interaction with atoms of object A bump into more atoms of object B until energy is transferred through all atoms of object B.
Schroeder defines Thermal Conduction as “transfer of heat by molecular contact: fast-moving molecules bump into slow-moving molecules, giving up some of their energy in the process” (Schroeder, 2007).
It is valuable to recall the three modes of heat transfer; convection for gases/liquids, radiation for objects separated by empty space and conduction for objects in direct contact.
Thermal conduction is also separated into three categories: molecular collisions for gas/liquid forms, lattice vibrations for solids and conduction electrons for metals as displayed in Figure 2. below.
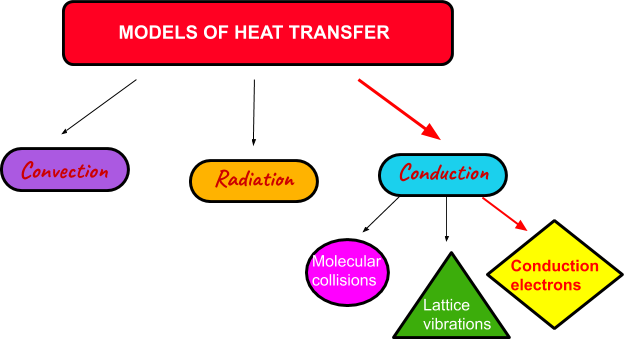
Figure 2. Modes of Heat Transfer.
Thermal Conduction of metals will include molecular collisions + conduction electrons for metals in gas state, and lattice vibrations + conducting electrons for metals in solid state. Conduction electrons are essentially what makes a metal an incredible conductor. Before explaining what a conduction electron really is, it is essential to recall the definition of a metal.
All elements can be found under the periodic table including Metals, Non-metals and Metalloids. Metals are defined as “elements that form positive ions by losing electrons during chemical reactions” (Blaber, 2015).
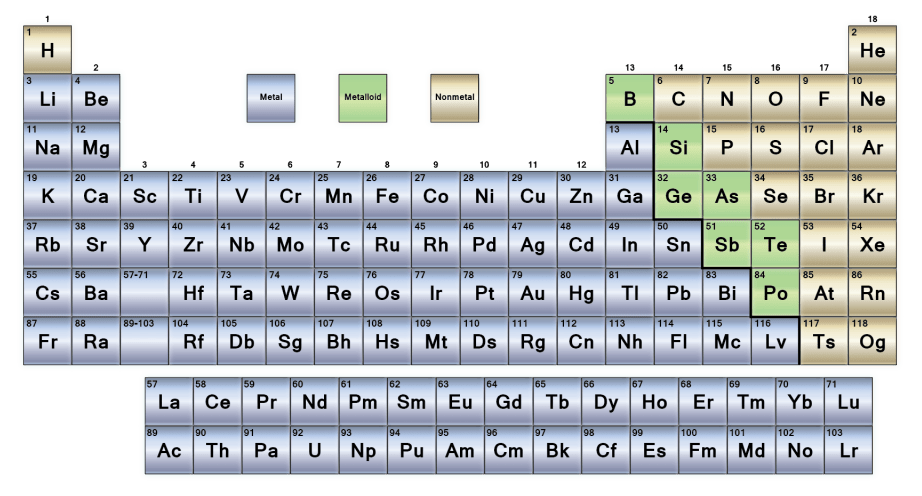
Figure 3. Periodic table showing all elements categorized into Metals, Non-metals and Metalloids.
Table 1. List of typical physical properties of Metals.
| Physical properties of most Metals |
|---|
| Solid at room temperature |
| Hard |
| High density |
| High Melting Point |
| High Boiling Point |
| Malleable |
| Ductile |
| Lustrous |
What makes a metal a good thermal conductor are the free-flowing conduction electrons.
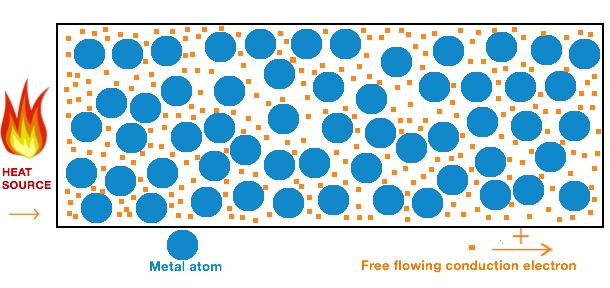
Figure 4. A metal block that is heated up displaying the atoms and the free flowing electrons.
Metal atoms shed valence electrons when chemically reacting with non-metal atoms, e.g. forming oxides and salts. Thus, metal ions are cations in an aqueous solution. What makes metals and metal alloys good conductors is the special metallic bonding. In metal solids, the bonded atoms share their valence electrons, forming a sea of freely moving conduction electrons which carry both heat and electrical charge. So, unlike e.g. electrons in covalent bonds, the valence electrons in a metal can freely flow through the metal latices, efficiently carrying heat without being locked to an individual atomic core.
Thermal conductivity (k) measures the ability of an entity to conduct heat (Q).
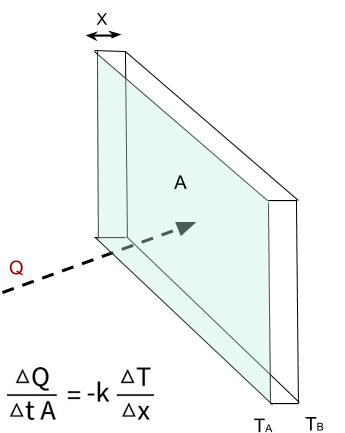
Figure 4. A sheet of material with the thermal conductivity equation.
Given:
k = thermal conductivity (W/m•K),
ΔQ = energy transfer (Joules/second),
Δt = change in time (seconds),
ΔT = temperature gradient (K),
A = area of thermal conductivity(m2),
Δx = thickness of material.
Table 2. List of typical physical properties of Metals.
| Metals | Thermal Conductivity at Room Temperature (W/m•K) |
|---|---|
| Aluminum | 226 |
| Carbon Steel | 71 |
| Magnesium | 151 |
| Brass (Yellow) | 117 |
| Bronze (Aluminum) | 71 |
| Copper | 397 |
| Iron | 72 |
| Stainless steel (446) | 23 |
| Tungsten | 197 |
| Lead | 34 |
| Nickel | 88 |
| Steel carbon type 1020 (0.2 – 0.6 c) | 71 |
| Zinc | 112 |
| Titanium | 21 |
| Tin | 62 |
Note: Copper and Aluminum have the highest thermal conductivity value (k). Check our material database.
Metals and alloys (materials made of a combination of metals) have uses as building materials in different industries such as electronics, engineering, laboratory equipment, medical devices, house-hold products, and construction.
The highest thermal conductivity value for metals are found in Silver (-429 W/m•K), Copper (-398 W/m•K) and Gold (-315 W/m•K).
Metals are highly important in making electronics as they are good conductors of electricity. Copper, aluminum, tin, lead, magnesium and plastic are often used in making parts of phones, laptops, computers and automotive electronics. Copper is cost-efficient and used for electrical wiring. Lead is used for cable sheathing and making batteries. Tin is used for making solders. Magnesium alloys are used in production of new technology as it is lightweight. Plastic is used for making parts of electronics that must not conduct electricity and titanium is used to produce plastic.
Metals are also important in the engineering industry. Aluminum is often used in making automotive & plane parts and used as an alloy since its pure form is weak. Automobile casting is made of zinc. Iron, steel and nickel are common metals used in construction and infrastructure. Steel is an alloy of iron and carbon (and often other elements). Increasing the carbon content in steel creates carbon steel, which makes the material stronger but less ductile. Carbon steel is often used in building materials. Brass and bronze (copper alloyed with zinc and tin, respectively) have beneficial surface friction properties and are used for locks & hinges and frames of doors & windows respectively.
Lastly, traditionally light bulb filaments for fluorescent light are made of tungsten. However, these are being phased out since only about 5 % of the power is converted to light in a light source like this, the rest of the power is converted to heat. Modern light sources are often based on LED technology and semi-conductors.
In conclusion, the thermal conductivity of metals is very important for designing any structure. It is integral for the safety, efficiency and new innovations within industries. The conductor electrons are the mechanism behind high conductivity of metals in comparison to non-metal materials. However, the thermal conductivity value (k) can vary greatly amongst metals as well.
Schroeder, D. V. (2018). An introduction to thermal physics. India: Pearson India Education Services.
Materials Database – Thermal Properties. (n.d.). Retrieved from https://thermtest.com/materials-database
Aluminum Alloys 101. (2020, March 9). Retrieved from https://www.aluminum.org/resources/industry-standards/aluminum-alloys-101
Elert, G. (n.d.). Conduction. Retrieved from https://physics.info/conduction/
Blaber, M. (2019, June 3). 9.2: Metals and Nonmetals and their Ions. Retrieved from https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_General_Chemistry_(Petrucci_et_al.)/09:_The_Periodic_Table_and_Some_Atomic_Properties/9.2:_Metals_and_Nonmetals_and_their_Ions
Thermal Conductivity. (n.d.). Retrieved from http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thercond.html
Titanium dioxide for Plastics. (n.d.). Retrieved from https://polymer-additives.specialchem.com/centers/titanium-dioxide-for-plastics-center
Sandhana, L., & Joseph, A. (2020, March 6). What is Carbon Steel? Retrieved from https://www.wisegeek.com/what-is-carbon-steel.html
(n.d.). Retrieved from http://www.elementalmatter.info/element-aluminium.html
Image 1.A: Mohamed, M. (2019). Cooking Lady [Illustration]. Retrieved from https://pxhere.com/en/photo/1584957.
Image 1.B: Mohamed, M. (2019). Chef Cooking [Illustration]. Retrieved from https://pxhere.com/en/photo/1587003.
Image 2: Wikipedia. Thermometer [Illustration]. Retrieved from https://upload.wikimedia.org/wikipedia/commons/7/70/Thermometer_CF.svg
Author: Selen Yildir | Junior Technical Writer | Thermtest