
January 23, 2024
In a world increasingly reliant on portable and renewable energy, Lithium-ion (Li-ion) batteries stand at the forefront of the technological revolution. From powering the smartphone in your pocket to driving the latest electric vehicles, Li-ion batteries are the unsung heroes of our modern lifestyle. But what makes these batteries so indispensable, and how do they function? Let’s dive into the fascinating world of Li-ion batteries, uncovering the science behind their operation, environmental impact, and pivotal role in shaping a sustainable future.
Join us on a journey through the heart of one of today’s most crucial and innovative technologies.
Primary cell batteries [1] are commonly used in everyday households and are non-rechargeable. These batteries are portable and inexpensive, but they have a limited lifetime. Every year, 15 billion non-rechargeable batteries are produced and sold worldwide [2]. These single-use batteries get tossed in the trash upon complete discharge. However, since they contain toxic metals and reactive acids, they are classified as hazardous waste and are harmful to the environment.
Currently, most of our portable electronic devices such as laptops and mobile phones come equipped with rechargeable Li-ion batteries. However, most people use disposable batteries in household items such as remote controls, flashlights, thermometers, weighing scales, and calculators utilize AA and AAA batteries.
The science behind batteries is fascinating – they operate by converting chemical reactions into electrical power through two terminals: one positive and one negative.

Figure 1. Types of primary cell batteries
With the rising concerns over sustainability issues and increasing attention towards nonrenewable sources of energy, research in long-lasting rechargeable batteries led to the development of Lithium-ion (Li-ion) batteries. In 2019, Akira Yoshino, John B. Goodenough, and M. Stanley Whittingham were awarded the Nobel Prize in chemistry for their pioneering work in Li-ion battery technology development, outlining the degree of impact Li-ion batteries made in the recent technological advances.
In Li-ion batteries, the movement of Li+ ions in electrolytes and electrons in an external current circuit generates electricity. It is vital to ensure this moment of electrons and ions happens in the designated paths.
A Li-ion battery has three major components:
A negative electrode is often called an anode, but this term is only applicable and correct during discharging. Similarly, during discharging, a positive electrode is correctly called a cathode.
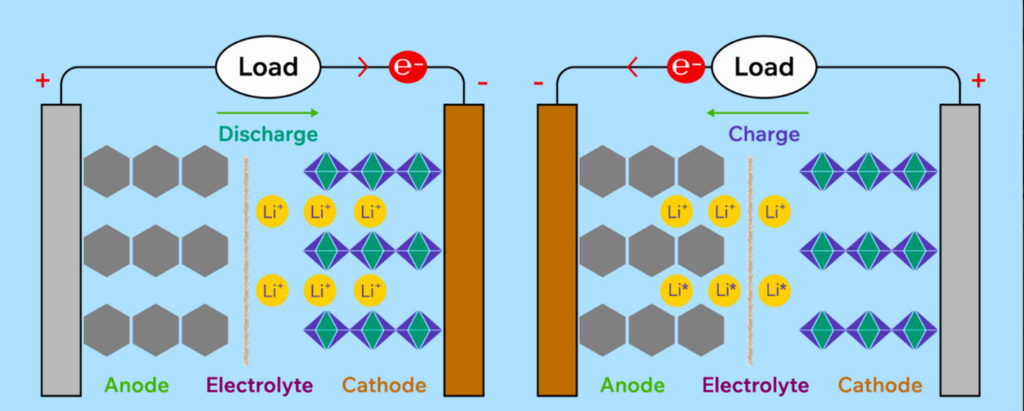
Figure 2. Mechanism of charge and discharge process in Li-ion batteries.
During charge, Li-ions move from the negative electrode to the positive electrode internally. In contrast, the electrons move from negative to positive electrodes through an external circuit to balance the charge. When you use a cell phone or drive an electric vehicle, the lithium-ions move from the positive to negative electrodes inside the battery while the electrons flow through the external circuit. For the process to work correctly, the electrolyte must permit only lithium ions to pass through it. At the same time, the external circuit made of copper or aluminum must allow only electrons to flow. The electrolyte must act as an electric insulator and be an excellent lithium-ion conductor.
Rechargeable li–ion batteries are currently the most viable alternative to fossil fuels for energy needs. The most fundamental unit of the battery is known as a cell. Battery cells typically come in different configurations, namely cylindrical, prismatic and pouch cells. Many such cells are connected in series and parallel configurations to complete a module. Multiple modules are connected to form a battery pack, which is used as a power source in various applications. This mechanism powers energy-intensive miniature devices like mobile phones and enables the technological advancement of electric vehicle manufacturing.
The critical components of a cyclical Lithium-ion cell are listed below in Figure 3. The battery is made by rolling the three sheets around the central core (Aluminum or Steel). Standard cell voltage is between 3V and 4.2V.
Electrons flowing from the negative to the positive electrode generate electricity. Li-ion batteries work on an interesting concept known as electrochemical potential in metallic materials. Electrochemical potential describes the tendency of a metal to lose or gain electrons. A positive value indicates a tendency to lose electrons, and a negative value indicates the tendency to gain electrons.
Among all the metals, Lithium has the highest propensity to lose electrons with an electrochemical potential of +3.04 V. Pure Lithium is highly reactive. Still, it becomes stable when inserted into a metal oxide, a sulfate, or a phosphate.
Inserting Lithium into a layered host is called intercalation, like hosting a guest ion or atom.[3] As depicted in Figure 4, transition metal oxides, sulphides and phosphates are used as cathode materials owing to their high cell voltages (3-5V), specific capacities (100-200 mAh/g)) and energy densities [4].
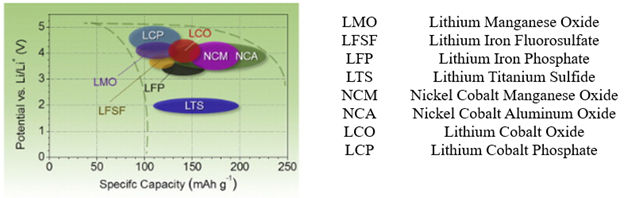
Figure 4. A range of cathode material cell chemistry is used in Li-ion batteries.
Li-ion batteries typically use Graphite as the negative electrode. This carbon form has been the choice anode material in commercial applications of Li-ion batteries for the last twenty years. The role of anode (or negative electrode) is to receive the Lithium ions during discharge and release them during charge. In intercalation, Lithium ions are inserted between the layers of carbon in Graphite. 6 Carbon atoms can host one Lithium atom [5]. Examples of alternative anode materials under investigation include Lithium Titanium Oxide (LTO), carbon nanotubes, and silicon [4,5].
The heat generated in batteries during charging/discharging can be detrimental to battery life and, if unchecked, can lead to catastrophic failure, known as thermal runaway. The thermal conductivity of Li-ion batteries significantly impacts battery temperature distribution, making thermal conductivity measurements crucial for designing efficient thermal management systems to improve cell life and prevent thermal runaway outbreaks.
Due to the inhomogeneous internal structure, batteries exhibit directional thermal conductivities along in-plane and out-of-plane directions. This property is known as anisotropy. Batteries’ in-plane and out-of-plane thermal conductivities are measured using the MP-1 Transient Plane Source (TPS) method with Anisotropic Module.
Transient methods measure temperature changes with time to study thermal transport properties such as thermal conductivity and thermal diffusivity. The TPS method is a transient thermal conductivity measurement technique Gustavsson et al. developed [8]. TPS method is standardized in compliance with international standards (ISO 22007-2) [9]. The TPS method places a planar heat source in direct contact with the test subject. The sensor consists of a thin nickel foil etched over an electrically insulating material like Kapton or Mica.
The sensor generates a heat pulse, and as the heat propagates into the sample, the temperature rise on the sensor surface is recorded over time. The increase in temperature is directly related to the sample’s thermal conductivity. The thermal conductivity and diffusivity can be calculated from the measured temperature rise by applying Fourier’s heat conduction theory. TPS measurements are conducted using the Measurement Platform Advanced (MP-1). The MP-1 is a laboratory instrument compatible with a range of sensors designed to perform robust measurements of thermal transport properties. It includes software capable of data acquisition and transient analysis.

Figure 5. Thermtest’s proprietary MP-1 and state-of-the-art Temperature Platforms.
The first step in the thermal property measurement of batteries is specific heat measurements. The pouch cell-specific heat testing is carried out in a specially designed thermally insulative sample holder. The experimental setup is shown in Figure 6.
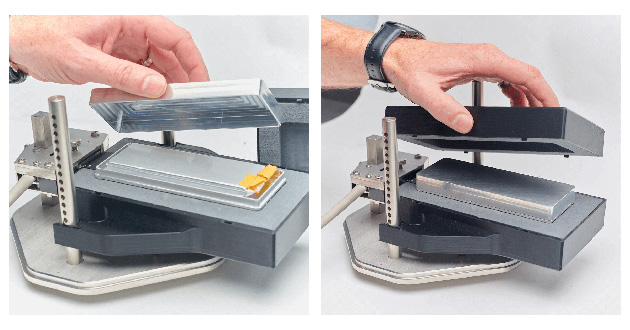
Figure 6. Set up for pouch battery-specific heat testing.
For testing the thermal conductivity and thermal diffusivity of pouch batteries, TPS 3D anisotropic module is used. Pouch batteries exhibit distinct thermal properties in different directions. The anisotropic module enables thermal conductivity measurement in two different orientations (radial and axial). Specific heat of the pouch battery is required to perform measurements in the Anisotropic module. The experimental setup for testing the thermal properties of pouch batteries is shown in Figure 7.
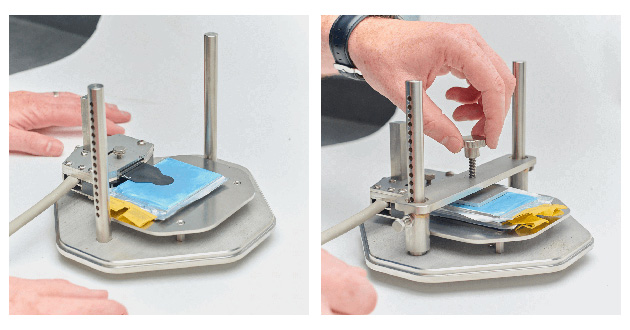
Figure 7. Experimental setup for cylindrical battery testing using the Anisotropic Module. With reference to sensor-sample orientation, the axial/in-plane direction is into the battery core, and the radial/out-of-plane direction is along the length of the battery.
Thermtest’s custom-designed battery enclosure can be used for measuring both in-plane and out-of-plane thermal conductivities of cylindrical batteries. Here, we measure the thermal conductivity and diffusivity of type 21700 cylindrical battery using the anisotropic TPS module.
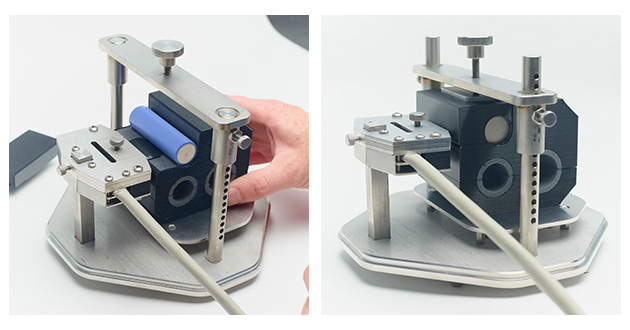
Figure 8. Experimental setup for cylindrical battery testing using the Anisotropic Module.
In over two decades, Li-ion batteries have not only fueled the wireless revolution but also significantly propelled the transportation sector into a new era. These batteries are integral in harnessing fluctuating renewable energy sources, like wind and solar, thereby offering a promising solution for grid-free electricity access. This blog underscores the critical role of thermal management and the measurement of thermal conductivity in optimizing Li-ion battery performance.
To explore more about our innovative approaches in thermal conductivity measurement and how they contribute to advancing Li-ion battery technology, we invite you to connect with our experts and discover our range of services at Thermtest.
[1] “Electric Battery,” Wikipedia [Online]. Available: https://en.m.wikipedia.org/wiki/Electric_battery.
[2] Lee, C.-H., Cheng, H.-W., Liao, B.-W., and Jiang, J.-A., 2022, “An Approach to Recover Energy From Discarded Primary Batteries Before Being Disassembled,” IEEE Transactions on Industrial Electronics, 69(6), pp. 6247–6257.
[3] Manthiram, A., 2020, “A Reflection on Lithium-Ion Battery Cathode Chemistry,” Nat Commun, 11(1), p. 1550.
[4] Nitta, N., Wu, F., Lee, J. T., and Yushin, G., 2015, “Li-Ion Battery Materials: Present and Future,” Materials Today, 18(5), pp. 252–264.
[5] Goriparti, S., Miele, E., De Angelis, F., Di Fabrizio, E., Proietti Zaccaria, R., and Capiglia, C., 2014, “Review on Recent Progress of Nanostructured Anode Materials for Li-Ion Batteries,” Journal of Power Sources, 257, pp. 421–443.
[6] Hossain, E., Murtaugh, D., Mody, J., Faruque, H. M. R., Haque Sunny, Md. S., and Mohammad, N., 2019, “A Comprehensive Review on Second-Life Batteries: Current State, Manufacturing Considerations, Applications, Impacts, Barriers & Potential Solutions, Business Strategies, and Policies,” IEEE Access, 7, pp. 73215–73252.
[7] Zhao, H., Yang, F., Li, C., Li, T., Zhang, S., Wang, C., Zhang, Z., and Wang, R., 2023, “Progress and Perspectives on Two-Dimensional Silicon Anodes for Lithium-Ion Batteries,” ChemPhysMater, 2(1), pp. 1–19.
[8] Gustafsson, S. E., 1991, “Transient Plane Source Techniques for Thermal Conductivity and Thermal Diffusivity Measurements of Solid Materials,” Review of Scientific Instruments, 62(3), pp. 797–804.
[9] “ISO 22007-2:Plastics — Determination of Thermal Conductivity and Thermal Diffusivity — Part 2: Transient Plane Heat Source (Hot Disc) Method.”
[10] “Axial and Radial Thermal Conductivity Measurement of 18,650 Lithium-Ion Battery – ScienceDirect” [Online]. Available: https://www-sciencedirect-com.proxy.hil.unb.ca/science/article/pii/S2352152X23019138. [Accessed: 03-Nov-2023].