
September 26, 2019
The forces behind thermal conductivity and how it is applied
Heat transfer is one of the main physical forces driving all reactions on this planet. Governed by the laws of thermodynamics, heat transfer enables the energy to be used and applied to power countless everyday systems. The mechanism of heat transfer is explained by the first law of thermodynamics. This law states that energy can neither be created nor destroyed, only transferred between systems.
Inevitably, when energy is transferred between two systems, some is lost to the surrounding environment. This lost energy occurs in the form of heat and can also be called thermal energy. Thermal energy contained within a system is responsible for the environment’s temperature.
The fundamental process where thermal energy is transferred from a warmer area to a cooler one within a material is referred to as heat conduction. Heat conduction is driven by the temperature difference between the areas. This transfer occurs as the kinetic energy of vibrating particles (atoms or molecules) is passed from one to another, moving the heat through the substance until a uniform temperature is achieved.
The efficiency of heat conduction varies with the material’s thermal conductivity—the property that defines how well a material can conduct heat. Metals, for example, are excellent heat conductors with their high thermal conductivity. However, materials like wood and air have low thermal conductivity and are excellent insulators.
Heat conduction has many applications, such as cooking with metal pans and insulating homes to prevent heat loss. It is critical in various fields of engineering and science because it allows controlling the heat transfer rate, which is vital for system design and thermal management.
There are three methods that facilitate heat transfer known as:
Radiation transfers heat using electromagnetic waves and does not involve any interaction between matter. The heat that comes from the sun is an example of radiation.
Convection occurs in liquids and gasses and describes the movement of heat from one location to another facilitated by the movement of fluids. When heated, fluids expand and become less dense. The hot fluid rises and displaces the cold fluid above it, pushing it toward the heat source. This cold fluid will become heated and rise upwards, creating a constant flow of fluid from an area of high heat to low heat. Convection explains how baseboard radiators can heat a whole room. The hot air generated from the radiators flows quickly upwards, pushing the cold air down towards the heater on the floor, creating a constant airflow. See more examples of convection.
Heat transfer through conduction involves the transfer of heat between two materials from surface contact. No matter is exchanged between materials, only energy. This type of heat transfer occurs in solid materials and is caused by particle vibrations. When exposed to a flow of energy, the particles in a solid begin to wiggle, rotate and vibrate, creating kinetic energy.
A common example of conduction is heating a pan on a stove. The heat from the burner transfers directly to the pan’s surface. Temperature measures the amount of kinetic energy processed by the particles in a sample of matter. The more kinetic energy a material has, its internal temperature will be higher.
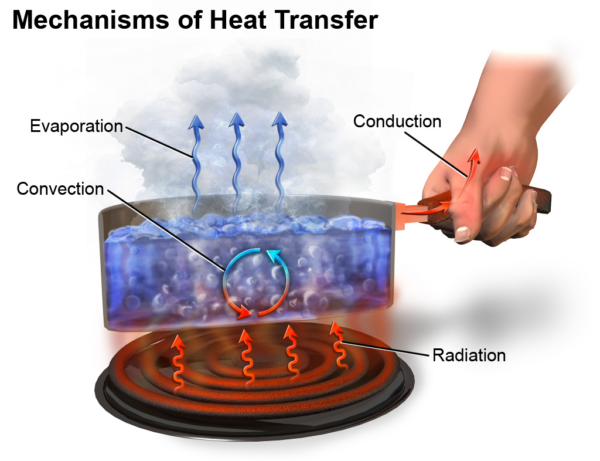
Figure 1: Mechanisms of heat transfer diagram
Matter with high kinetic energy will also have a high thermal conductivity. Thermal conductivity describes how efficiently a material can pass heat through it. It is defined by the rate of energy flow per unit area when compared to a temperature gradient. Most conductivity values are expressed in Watts per meter per degree Kelvin W/m•K.
Thermal conductivity explains why walking barefoot on a cold tile floor feels much cooler than walking on carpet, even though both are at room temperature. Tile and rocks have a higher thermal conductivity than carpet and fabrics, so they can transfer heat away from a foot at a much quicker rate, making the tile appear cool to the touch.
Metals are an example of a material with a high thermal conductivity that can quickly transfer heat. The internal structure of a metal molecule contains free electrons that can move freely through the bulk of the material. These free electrons collide rapidly with other particles, causing a metal’s internal structure to vibrate faster and heat up quicker. These rapid vibrations promote energy and heat flow throughout the metal.
Metals like copper, aluminum and silver are frequently used to make thermal appliances and tools. Copper pipes are wires that are extremely popular to use within a home to transfer energy and heat quickly from one area to another. Aluminum has extremely similar thermal properties to copper and is often used as a cost-effective replacement to perform the same functions.
Silver is one of the most widely used metals for thermal applications. Over 35% of all silver produced in the USA is manufactured for electronics or electrical uses. The demand for silver continues to grow as it is becoming a crucial component in producing solar panels. Other highly thermally conductive materials, such as diamonds, also have many practical applications. Diamond powder is often used in electronics to transfer heat away from sensitive areas to protect them from overheating.

Figure 2: Standard solar panels that are frequently manufacture with silver
Non-metal materials rely on phonons to transfer heat along a gradient from cold to warm areas. Plastics, foams, and wood are all examples of materials with poor thermal conductivity values. These materials are known as insulators and can restrict the flow of heat. Insulators have numerous extremely useful applications that can protect energy from being lost to the environment.
Foam is an extremely useful home and building insulation material. Over 50% of all household energy is used to heat or cool a home. Using a high thermal conductive material to insulate a house can substantially lower the energy required to heat or cool a building. Energy prices continuously increase globally, making it ideal to conserve as much power and heat as possible to lower power bills.
Thermal conductivity is an extremely important material property that enables thousands of production systems to function properly and efficiently. Heat is constantly being exchanged within every ecosystem in the form of lost energy. Harnessing thermal energy for industrial and practical processes has created excellent energy-saving technologies that are utilized daily.
Heat can move through a system through conduction, radiation, and convection. Structure, density and material composition can impact a sample’s thermal conductivity. Materials with high or low thermal conductivity values are used for various everyday applications. Although highly underestimated, life would not be the same without heat transfer and thermal exchange.
For a deeper understanding of heat transfer mechanism, refer to the basics of heat transfer.
Shindé, S., & Goela, J. (2006). High thermal conductivity materials. New York: Springer. doi:10.1007/b106785]
The Physics Classroom Tutorial. (n.d.). Retrieved from https://www.physicsclassroom.com/class/thermalP/Lesson-1/Methods-of-Heat-Transfer
What is thermal energy? (n.d.). Retrieved from https://www.khanacademy.org/science/physics/work-and-energy/work-and-energy-tutorial/a/what-is-thermal-energy
Featured Image: https://unsplash.com
Author: Kallista Wilson | Junior Technical Writer | Thermtest