August 19, 2024
Everything you need to know about specific heat capacity, its importance in thermodynamics, and how it differs from heat capacity. Explore examples, formulas, and frequently asked questions to deepen your understanding.
This concept is fundamental to thermodynamics and heat transfer, since it expresses the amount of heat energy that must be supplied to a unit of mass of a substance to raise its temperature by one degree Celsius. This concept has several important applications in practice. Engineers and materials scientists use it to choose the right materials that will bring rapid temperature changes where needed and ensure thermal stability where necessary.
It is the amount of heat energy required to raise any substance’s temperature by one degree Celsius. This is an intrinsic property of the material and depends upon the nature of the substance. It is one of the most critical attributes for scientists and engineers because it touches areas from climate science to culinary arts. 1
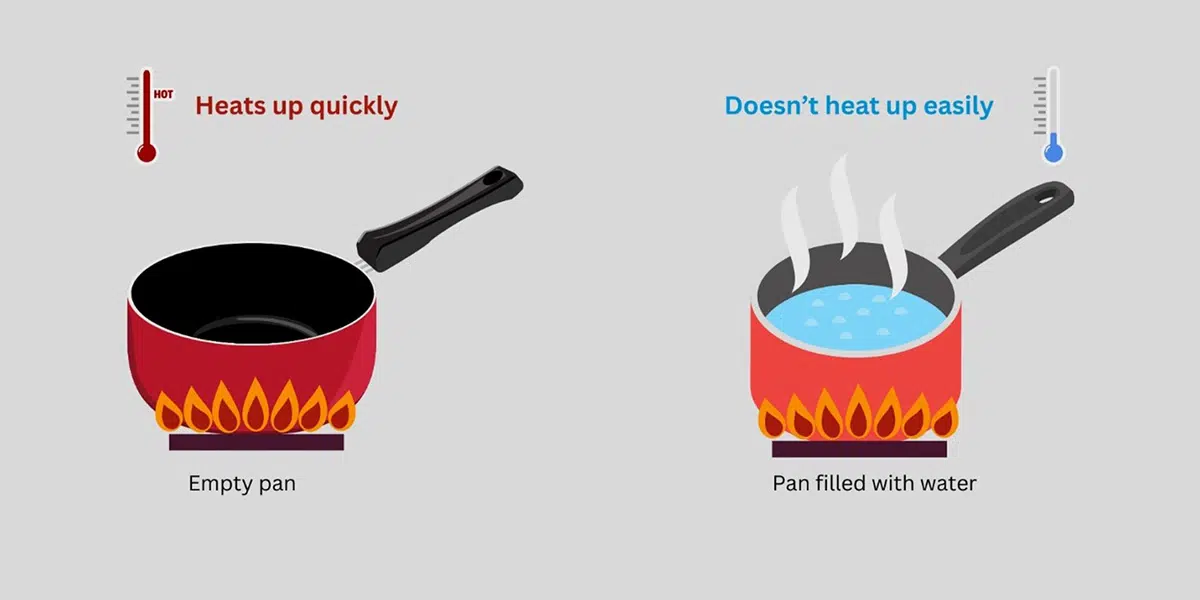
Understanding this characteristic means that different materials, upon receiving the same amount of heat, change temperature by different amounts. This is due to the molecular structure and bonding nature of each substance. Imagine you are at the beach on a hot day. The sand feels extremely hot, while the water does not. It is because sand has a low specific heat, it warms relatively quickly. On the other hand, water’s high specific heat allows it to absorb more heat without raising its temperature as quickly.
It is defined as the amount of heat energy required to increase the entire object’s temperature by one degree Celsius. Heat capacity does depend upon the mass of an object and its composition.
For example, while specific heat for both a large block of iron and a small iron nail is the same; heat capacity will be different as the large block of iron will need more heat to raise the temperature by one degree while the small iron nail would need relatively less heat to achieve the same temperature increase.
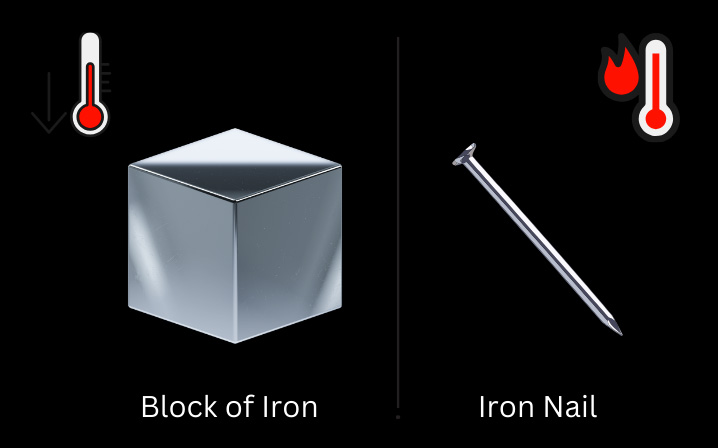
Figure 1: An Iron nail will heat up more quickly than an iron block with the same amount of heat showcasing heat capacity theory.
The unit of specific heat capacity in the International System of Units (SI) is joules per kilogram per Kelvin (J/kg·K). The equation used to calculate specific heat capacity (c) is:
c=Q/mΔT
Where:
This formula is derived from the relationship between thermal energy and temperature change, emphasizing how the specific heat capacity moderates this relationship.
Specific Heat at Constant Pressure (cp): This is the change in internal energy with respect to the change in temperature at a fixed pressure.
Specific Heat at Constant Volume (cv): This is the change in internal energy with respect to the change in temperature at a fixed volume.1
For most solids and liquids, the work done by pressure can be neglected because their volume does not change significantly with temperature. Thus, for these substances, cp and cv are approximately equal. 2
Specific heat capacity for gases is more complicated than for solids and liquids. Gases expand significantly when heated, so it depends on whether the pressure or volume is held constant during the heating process. For example:
Steam (at 100°C):
Therefore, understanding these differences when designing engines or other machinery that use gases ensures that the equipment can handle thermal stress and operate efficiently.
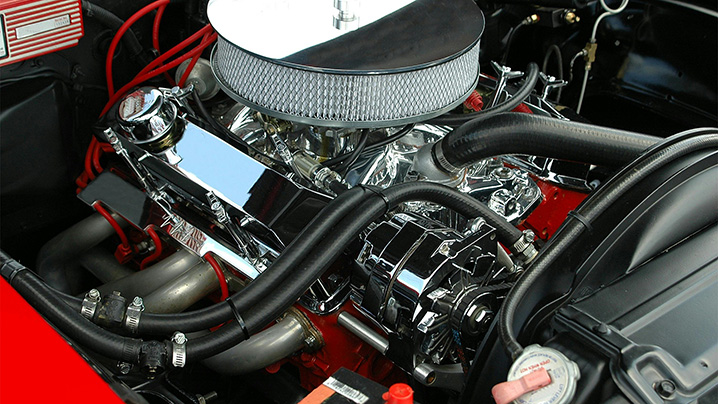
Figure 2: Car engines are designed considering the specific heat of the gases involved in combustion to prevent overheating and maximize performance
Water (liquid) 4.18 J/kg/C: Water has great specific capacity, which is one of its best qualities since it helps it to conduct heat effectively. It can absorb and release massive amounts of energy without undergoing much variation in temperatures. For instance, car radiator systems and electricity generation amid other industries. 4
Steam 2.01 J/kg/C: The specific heat of steam is important in the design of steam engines and turbines. Since engineers must know how much energy is needed to make steam, and they also must know how much heat can be carried by the steam, they shall view power generation systems in relation to their efficiency.
Copper 0.385J/kg/C: The specific heat capacity of copper applies to both electrical engineers and plumbers because it can efficiently conduct heat. This property is very important when creating any electrical wiring or electronic circuit boards that involve heat exchange. This ensures the proper management of temperatures within an electronic device and system.
Iron 0.449J/kg/C: Iron’s specific heat capacity is an important factor not only in the manufacturing of cookware like pans and skillets but also in the construction industry. This property allows iron to effectively absorb and retain heat, making it valuable for creating materials that require durability and thermal stability.
Test example: Specific Heat Capacity Test: The Method of Mixture
For many scientific and engineering applications, it is important to understand specific heat capacity and its relationship with regards to heat capacity. The main messages that could be drawn from this discussion are below.
Therefore, it is a fundamental concept that connects theoretical understanding to practical applications. It plays a critical role in everything from industrial machinery to household appliances, enabling informed decisions about performance, safety, and energy efficiency. To ensure you’re making the best choices, it’s vital to have accurate instruments to measure thermal properties. Explore Thermtest’s product line to find the right tools for your needs.
Specific heat depends on the material’s molecular structure and bonding. Substances with stronger molecular bonds typically have higher specific heat because more energy is required to increase their temperature.
The specific heat capacity of water at room temperature and pressure is approximately 4.18 J/g°C.
Water has one of the highest specific heat capacities among common substances, at 4.18 J/g°C. This is because of the hydrogen bonding between water molecules, which requires significant energy to overcome. 5
It can be measured using a calorimeter, which quantifies the amount of heat transferred to or from a substance as its temperature changes.
Heat Capacity measures the heat required to raise the substance’s temperature by 1 degree. On the other hand, specific heat capacity is the amount of heat required to raise the temperature of 1 kg of a substance by 1 degree. While the former depends on the total mass or the amount of substance, the later does not. That is why the concept of heat capacity is used to understand how much an object can absorb or release heat during a given temperature change, and the concept of specific heat capacity is helpful in relating thermal properties of different materials.
The SI unit is joules per kilogram per Kelvin (J/kg·K).
This can be test using different methods such as LFA, DSC, TPS and THW techniques. Check our testing services for more info.
1Giancoli, D. C. (2013). Physics: Principles with Applications (7th ed.). Pearson Education.
2Heat, Work and Energy. (2003). Retrieved from The Engineering ToolBox : https://www.engineeringtoolbox.com/heat-work-energy-d_292.html
3Mosca, P. A. (2008). Physics for Scientists and Engineers (6th ed.). New York: W. H. Freeman and Company . Retrieved from • Tipler, P. A., & Mosca, G. (2007). . W.H. Freeman and Company. Pg: 606-611
4Specific Heat Capacity and Water. (2018, June 06). Retrieved from Water Science School: https://www.usgs.gov/special-topics/water-science-school/science/specific-heat-capacity-and-water
5Thermal Energy. (2024, July 2). Retrieved from Examples.com: https://www.examples.com/physics/thermal-energy.html
6Why is Specific heat of water high? (2009). Retrieved from Stack Exchange: https://chemistry.stackexchange.com/questions/26651/why-is-the-specific-heat-of-water-high