
December 13, 2024
Phase change materials (or PCMs) are materials that absorb and release large amounts of energy when they change phases, for example from solid to liquid or liquid to gas, to provide the stored energy for heating or cooling a system . In most cases, the change of matter happens between solid to liquid.
The material melts or solidifies at the phase change temperature (PCT), and by doing so a PCM is capable of absorbing or releasing a substantial amount of energy as compared to normal. Energy is stored or released by changes of state or by a change in the internal structure – this is why PCMs are also called latent heat storage (LHS) materials.
Latent heat storage using PCMs finds application in buildings, energy storage systems, waste heat recovery systems, thermo-regulating fibers, smart textile materials, thermal management of batteries, temperature management of the microelectronics, photovoltaic thermal (PV/T) applications, space and terrestrial thermal energy storage applications, and in the temperature management of greenhouses.
There are 3 types of phase change materials: Organic PCMs, Inorganic PCMs, and Eutectic PCMs.
1. Organic phase change materials (PCM) are most commonly made of hydrocarbon-based substances. There are two subcategories: paraffin compounds or fatty acids .

Figure 1: Sample of paraffin wax
Some of the advantages and disadvantages of organic PCM are:
Advantages:
Disadvantages:
2. Inorganic PCMs: Inorganic PCMs are commonly fabricated from salt hydrates or metals . They are distinctly efficient in terms of energy storage and have better thermal conductivity as compared to organic PCMs.
Some advantages and disadvantages of inorganic PCMs are:
Advantages:
Disadvantages:
3. Eutectic PCMs: They combine two or more organic or inorganic PCMs. There are three types of Eutectic PCMs:
Some advantages and disadvantages of eutectic PCMs are:
Advantages:
Disadvantages:
Here are some examples for various PCMs:
| Name | Types | Composition | Melting Point |
| Paraffin Waxes |
Organic
|
Hydro-carbon chains | Between 20°C and 60°C |
| Fatty Acids | Organic | Carboxylic acids | Between 20°C to 50°C |
| Salt Hydrates | Inorganic | Inorganic salt with water molecules | Varies widely depending on the specific salt and its hydration level |
| Eutectic Mixtures | Mixture of two or more salts | Customized based on the mixture ratio |
PCMs use latent heat absorbed or released during their phase transition. Let’s break it down further:
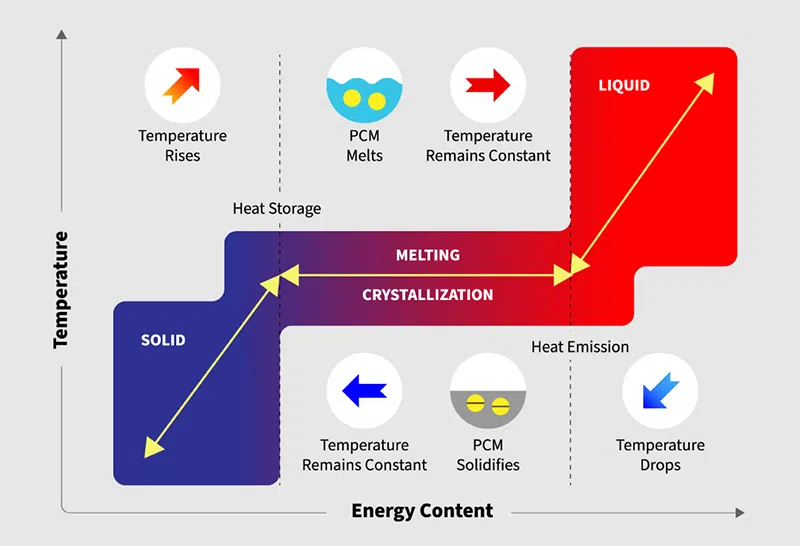
Figure 2: Working of phase change materials
Absorbing heat: When a PCM gets warmer, it reaches a certain temperature where it starts to change state (like melting from solid to liquid). At this point, the PCM takes in a lot of heat without getting much hotter. This heat helps break the bonds between the particles in the solid, turning it into a liquid.
Storing energy: After the PCM fully becomes a liquid, it can keep this hidden heat for a long time, giving a reliable and efficient way to store thermal energy. For example, PCMs can soak up heat from the sun during the day and store it and release it when the surroundings get cold.
Releasing heat: The environment cools down, slowly solidifying the PCM making it release the stored heat. This heat is then used in various applications to maintain the temperature. For example, it helps to keep the building warm during cold days.
PCMs can be used in many different industries because they are so flexible. Here are some of the most common uses:
Buildings: PCMs are used in walls, floors and ceilings to maintain indoor temperatures. They absorb heat during the day and release it at night. For example, paraffin wax and salt hydrates are used in building materials.
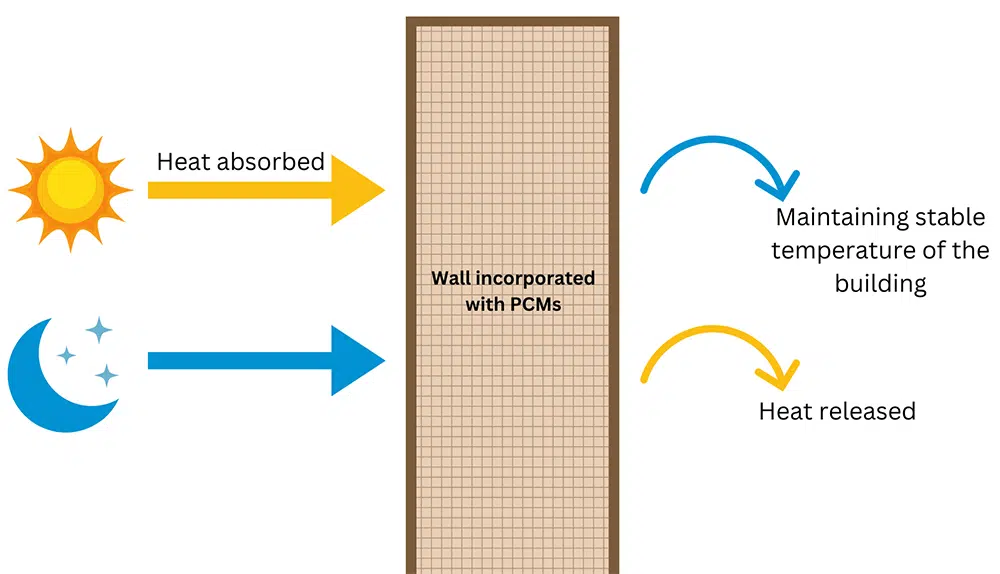
Figure 3: The walls insulated with PCMs helps to maintain a stable temperature of the building
Solar energy storage: PCMs work by storing extra solar energy collected during the day and releasing it during the night, making solar heating systems efficient. Sodium sulfate decahydrate is an example of PCMs used in solar energy systems.
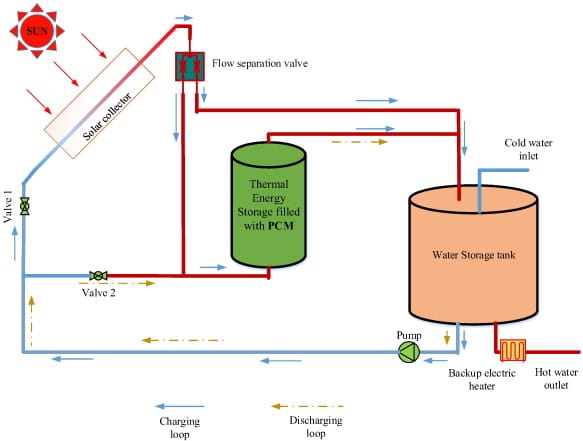
Figure 4: Layout of a solar water heater using PCMs
Refrigeration and cold storage: Refrigerators and cold storages have a lining of PCMs to ensure the temperature stays cold during power-off or during transportation. A mix of ammonium chloride and water is used to keep it cool.
Textiles: PCMs are added into clothing to help control body temperature, keeping the person wearing it warm or cool depending on the environment. Paraffin wax is most commonly used in textiles.
Heat pumps: The PCMs store excess heat generated during the heat pump’s operations. When the demand increases, the stored energy is released. The frequency of switching on and off reduces, helping to save energy and increasing the life of the compressor. Heat pumps most commonly use paraffin-based PCMs or salt hydrates due to their favorable thermal properties.
Explore more diverse applications of phase change materials.
Phase change materials are a major advancement in managing heat and improving energy efficiency. Their special feature of storing and releasing heat can be used in many ways to support sustainable methods in different fields. By learning about the types, compositions, and mechanisms of PCMs, we can better understand their importance in current technology and energy solutions.
Phase Change Materials are used for energy storage, regulating temperature and thermal management in various industries, including buildings, textiles and electronics.
PCMs absorb and store excess heat during warmer periods and release it during cooler periods, helping to maintain a stable temperature and save energy.
Most PCMs, especially organic ones like paraffin wax, are safe for everyday use. However, some inorganic PCMs, such as salt hydrates, can cause corrosion if not properly managed.
Yes, many PCMs like fatty acids and paraffin are recyclable and can be reused in multiple thermal cycles.
The lifespan of a PCM depends on the material and its application. Some of them can undergo thousands of phase change cycles without significant degradation.
Yes, Thermtest’s products can measure the thermal properties of Phase Change Materials (PCM), such as MP-V (Versatile Measurement Platform) and THW-L1. These instruments provide precise measurements of thermal conductivity, specific heat, and other important thermal properties, which are essential for analyzing PCM performance.
Kuta, M., Matuszewska, D. & Wójcik, T. M. The role of phase change materials for the sustainable energy. E3S Web Conf. 10, 00068 (2016).
Cui Y, Xie J, Liu J, Wang J, Chen S. A review on phase change material application in building. Advances in Mechanical Engineering. 2017;9(6). doi:10.1177/1687814017700828