Join us at the International Thermal Conductivity Conference (ITCC) and the International Thermal Expansion Symposium (ITES).
February 16, 2024
May 4, 2021
September 26, 2019
May 25, 2021
October 30, 2019

November 29, 2019
The thermal conductivity of a material or element is a defining property that aids in the development of effective heating/cooling technologies. A value for thermal conductivity can be determined by measuring the rate at which heat can pass through a material.
This value is expressed in Watts per meter per degree Kelvin W/m•K following the S.I. (International System) guidelines. Materials with a high thermal conductivity can effectively transfer heat, while materials with lower thermal conductivity do not readily transfer heat and are slow to take up heat from their surroundings.
Numerous chemical and physical properties of an element or material can influence its thermal conductivity. Generally, materials with a simple chemical composition and molecular structure have higher thermal conductivities. A common physical characteristic that can influence a materials thermal conductivity is porosity. Air has a thermal conductivity of 0.02 W/m•K at room temperature (20-25°C). This value is considerably lower than most solid matter. When air is trapped in the pores of a substance it can decrease the rate at which heat can effectively pass through it. Pore size, distribution, shape and connectivity all influence the porosity of a material. A high porosity will lower thermal conductivity. Other external factors that can impact thermal conductivity are humidity and direction of heat flow.
Water and ice both have a higher thermal conductivity than air. If a material is exposed to an environment with moisture present, it can potentially be absorbed and will yield a higher thermal conductivity value. A materials molecular structure also has the potential to restrict heat flow. Wood is an example of a material that has a molecular structure composed of straight fibers. Heat flowing through wood moves in a constant direction following the path provided by the fibers. If heat flows against fibers, there is greater resistance. This resistance can limit effective heat transfer and lower the thermal conductivity of that material.

Figure 1: Porosity displayed in a rock sample
Thermally conductive materials are substances that are capable of efficiently transferring heat. These materials have a high thermal conductivity, which means they can effectively conduct heat and transfer it from one area to another. This property makes them valuable for various applications in heating and cooling technologies.
Diamond currently holds the title for being the thermally conductive material known to man. A diamond’s thermal conductivity can reach as high as 2000 – 2200 W/m•K when measured at room temperature (20-25°C). This thermal conductivity value is almost 5x’s higher than silver which has the second highest conductivity value. Diamond is commonly used in electronics for heat dispersion to protect sensitive devices from overheating. Their high thermal conductivity values can also be used to detect the authenticity of diamonds in jewelry.
Contradictory to the electrons that transport heat in metals, photons transport energy and heat in diamonds. It is assumed by the scientific community that a diamonds surface is covered by a thin carbide film that assists in pairing electrons with photons. This interaction is currently being researched and appears to be the heat transfer medium that influences the elevated thermal conductivity of a diamond. The structure of the simple carbon bonds in diamonds also impacts the transportation of heat through each atom. As mentioned above, materials with a simple molecular structure often have a higher thermal conductivity.
Silver has the second highest measured thermal conductivity values. Silver is an abundant and relatively inexpensive metal that has been incorporated into thousands of practical appliances and technologies. 35% of silver processed in the United States is used for electronics and electrical purposes (U.S geological survey mineral community summary 2013). Silver is a relatively malleable metal that can easily be manipulated into a variety of viscosities and particle sizes. This property of silver has contributed to the wide spread use of the metal. Silver paste is an example of a manufactured product of silver that has a steadily increasing demand. Silver paste is frequently used in the production of photovoltaic cells, a major component of solar panels.
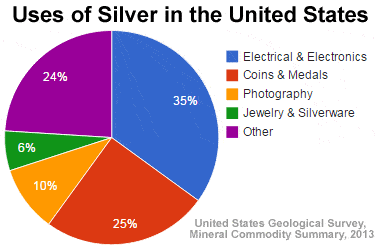
Figure 2: Pie chart displaying the uses of silver in the United States of America.
Copper is the material with the third highest thermal conductivity and is also the most popular metal to use for manufacturing conduction technologies. Copper is an extremely effective material for minimalizing energy loss during heat transfer. Copper has a high melting point and a slow corrosion rate. Sauce pans, hot water pipes and electronic heat sinks are examples of appliances that utilize copper’s thermal conductive properties.
Gold has similar conductive properties to copper but is rare and very costly to obtain. Gold doesn’t tarnish as quickly as copper and silver so is often used on electrical contacts and connecters due to its strong wear resistance.
Aluminum is another metal with an elevated thermal conductivity value. Aluminum has a relatively low melting point compared to other metals and is often used as a cost-effective alternative for copper. Copper-aluminum mixes are frequently manufactured to harness the chemical and physical characteristics of both metals and minimalize production expenses.
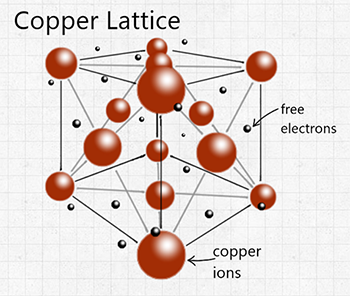
Figure 3: Lattice backbone of a copper atom
| Material | Thermal Conductivity W/m•K at (20-25°C) |
|---|---|
| Diamond | 2000-2200 |
| Silver | 429 |
| Copper | 398 |
| Gold | 315 |
| Aluminum Nitride | 320 |
| Silicon Carbide | 270 |
| Aluminum | 247 |
| Tungsten | 173 |
| Graphite | 168 |
| Zinc | 116 |
Table 1: Top 10 thermally conductive materials and their conductivity values measured in W/m•K at room temperature (20-25°C)
Thermal conductivity is a major component of minerals and elements that is critical to the engineering of countless technologies and tools. Selecting a material with the proper conductivity value can increase the efficiency of the product and save energy and money. The most exceptional thermal conductors are metals due to their internal electron arrangement. Metal ions are tightly packed in a lattice formation (figure 3). These ions are constantly vibrating which is generating heat.
The metals molecular structure also includes free electrons. These delocalized electrons carry large amounts of energy as they move through the lattice. As these electrons collide with the lattice backbone, they excite the ion structure causing it to vibrate more rapidly. The increased vibration of the lattice starts to generate more heat. Due to the movement of the free electrons present in metal, the heat generated from ion vibration can transfer through the substance more efficiently.
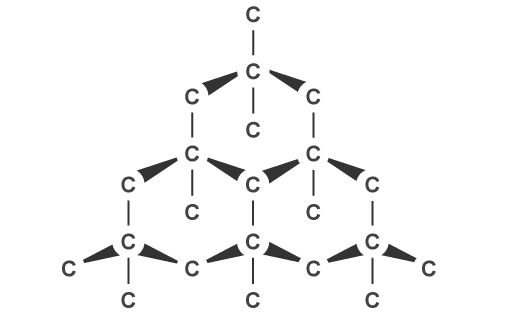
Figure 4: Lewis diagram displaying carbon backbone of a diamond atom
As the first law of thermal dynamics states, energy can neither be created or destroyed. Energy (heat) can only be transferred. Effective heat transfer requires efficient thermal conductors. Materials with high thermal conductivities are crucial in the engineering and development of countless heat transferring electronics and appliances. Every thermal conductor has unique chemical and physical properties that enable the heating properties to be utilized in a beneficial way.
Irimia R, Gottschling M (2016) Taxonomic revision of Rochefortia Sw. (Ehretiaceae, Boraginales). Biodiversity Data Journal 4: E7720. (n.d.). https://doi.org/10.3897/BDJ.4.e7720 .
Flickr. 1https://live.staticflickr.com/8227/8456671200_5c93da3b69_b.jpg
The Many Uses of Silver. (n.d.). Retrieved from 2https://geology.com/articles/uses-of-silver/
Electronics: The Very Basics. (2015, April 21). Retrieved from 3http://stuartparkinson.com/electronics-the-very-basics/
(n.d.). Retrieved from https://www.cs.mcgill.ca/~rwest/wikispeedia/wpcd/wp/m/Material_properties_of_diamond.htm
Copper Properties and Applications – electrical, thermal, corrosion resistance, alloying and more. (n.d.). Retrieved from https://copperalliance.org.uk/knowledge-base/education/education-resources/copper-properties-applications/
Diamond Structure. (1970, January 01). Retrieved from 4http://sciencesolve.blogspot.com/2015/09/diamond-structure.html
Author: Kallista Wilson, Junior Technical Writer at Thermtest